23 Open-label Clinical Trial
ClinicalTrials.gov is a resource provided by the U.S. National Library of Medicine. IMPORTANT: Listing a study does not mean it has been evaluated by the U.S. Federal Government.Read our disclaimer for details.. Before participating in a study, talk to your health care provider and learn about the risks and potential benefits. Open-Label Phase 2 Study of Ladiratuzumab Vedotin (LV) for Unresectable Locally Advanced or Metastatic Solid Tumors ... Clinical Trial Details. We are doing this study to find out if it works for solid tumor cancers and what kind of side effects it causes LV is a type of drug called an antibody drug conjugate or ADC. ADCs usually have 2 parts:
An open-label single-dose trial evaluated safety, pharmacokinetics, sensory gating, attention, impulsivity, and inhibition in 12 adult males and females. The single dose of fenobam was associated with a significant improvement of PPI compared to the untreated control group from a previous study (Berry-Kravis et al., 2009).
 Clinical trial for Myasthenia Gravis generalised | generalized myasthenia gravis | Myasthenia Gravis (Chronic Weakness) | Myasthenia Gravis , Open-Label Extension of Zilucoplan in Subjects With Generalized Myasthenia Gravis A Phase 1, Open-Label, Multicenter Study to Assess the Safety, Tolerability, Pharmacokinetics, and Preliminary Antitumor Activity of Ascending Doses of AZD5991 in Subjects with Relapsed or Refractory Hematologic Malignancies. Clinical Trial Details. For open‐label trials, this routinely used concept is referred to as prospective randomized open‐label blinded endpoint (PROBE) design. Therefore, the potential of a bias influencing the results is currently very low for double‐blind but also for open label anticoagulation trials.
Clinical trial for Myasthenia Gravis generalised | generalized myasthenia gravis | Myasthenia Gravis (Chronic Weakness) | Myasthenia Gravis , Open-Label Extension of Zilucoplan in Subjects With Generalized Myasthenia Gravis A Phase 1, Open-Label, Multicenter Study to Assess the Safety, Tolerability, Pharmacokinetics, and Preliminary Antitumor Activity of Ascending Doses of AZD5991 in Subjects with Relapsed or Refractory Hematologic Malignancies. Clinical Trial Details. For open‐label trials, this routinely used concept is referred to as prospective randomized open‐label blinded endpoint (PROBE) design. Therefore, the potential of a bias influencing the results is currently very low for double‐blind but also for open label anticoagulation trials.
Open-label clinical trial. Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population. Methods We conducted a noncontrolled, open-label clinical trial of azithromycin 250 mg once daily for 3 months in patients with pulmonary sarcoidosis who reported a chronic cough. The primary outcome was number of coughs in 24 h. Secondary outcomes were cough visual analogue scales and quality of life measured using the Leicester Cough Questionnaire and King's Sarcoidosis Questionnaire. Open Label Extension, or OLE, is a phase of a study that occurs after the randomized (blinded) portion of the trial is completed if a drug is found to have the potential for benefit. Eligible trial participants take the active form of the drug without placebo. OLE allows active drug to be given to all participants at the same time and to follow them over time. A Phase 2b/3, Multi-Center, Randomized, Double-Blind, Placebo-Controlled, 12 Month Clinical Trial to Evaluate the Efficacy and Safety of MN-166 (Ibudiast) followed by an Open-Label Extension Phase in Subjects with Amyotrophic Lateral Sclerosis. Status: Currently enrolling Principal Investigator: Nicholas J. Maragakis, M.D.
An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. In particular, both the researchers and participants know which treatment is being administered. CONSORT flowchart of recruitment and enrollment for the open-label phase of clinical trial NCT02548559. COVID-19 indicates coronavirus disease 2019. Table. Demographics, Creatinine Levels, Product Use, and Δ9-Tetrahydrocannabinol Metabolite Results Following 4 Weeks of Treatment With a Full-Spectrum, ... This Phase 1 open-label, multicenter, multidose, first-in-human clinical trial of SNX281 will evaluate the safety and tolerability of SNX281 alone and in combination with the PD-1 inhibitor pembrolizumab in subjects with relapsed or refractory solid tumors or lymphomas. The trial is designed to enroll up to 128 patients. Blinding Sponsors for Open Label Studies: Challenges and Solutions . Quan Jenny Zhou, Shengyan Hong, and Zhengping Ma . Eli Lilly and Company, Indianapolis, IN . ABSTRACT . Although double blinding (blind treating physicians and patients) is the optimal approach to minimize the bias in clinical research, it's not always feasible to conduct
Purpose: This study consists of a 6-week, open-label, randomized clinical trial study to compare efficacy and tolerability of the natural treatments omega-3 fatty acids, inositol, and N-acetylcysteine (NAC) in the treatment of mood dysregulation in children and adolescents (ages 5-17). Note: Auxiliary medicinal products should not include concomitant medications, that is medications unrelated to the clinical trial and not relevant for the design of the clinical trial. (CTR (whereas 54)). The Regulation states that IMP and AMP should be appropriately labelled to ensure; subject safety, reliability and robustness of data, and allow the distribution to clinical sites. l A one-arm study is an observational study—not a clinical trial or an experiment. ... – For non-masked (open label) studies this means before any patient reaches an outcome. 36. Data Monitoring Committees for Clinical Trial Sponsors, The Establishment and Operation of Clinical Trial - 03/2006 Data Retention When Subjects Withdraw from FDA-Regulated Clinical Trials - 10/2008
 What are clinical trials definition and examples market
What are clinical trials definition and examples market
A frequent feature of pharmaceutical research is the open label extension study, in which patients participating in double blind placebo controlled trials of new medications are invited, on completion of the initial trial, to take the study drug for some further period. Patients are openly given the active substance at this stage, regardless of their assignment in the initial trial.
 An open label cluster randomized controlled trial of
An open label cluster randomized controlled trial of
A phase 1 open-label clinical trial of the safety and tolerability of single escalating doses of autologous CD4 T cells transduced with VRX496 in HIV-positive subjects. MacGregor RR(1). Author information: (1)Division of Infectious Diseases, University of Pennsylvania Medical Center, Philadelphia, PA 19104, USA. macgregr@mail.med.upenn.edu
 Glossary of clinical trials terms
Glossary of clinical trials terms
Open-label extension studies do have a legitimate but limited place in the clinical development of new medicines. The negative perceptions about these studies have arisen because of perversion of acceptable rationales for this type of study and a failure to recognise (or disclose) the limitations resulting from the inherent weaknesses in their design.
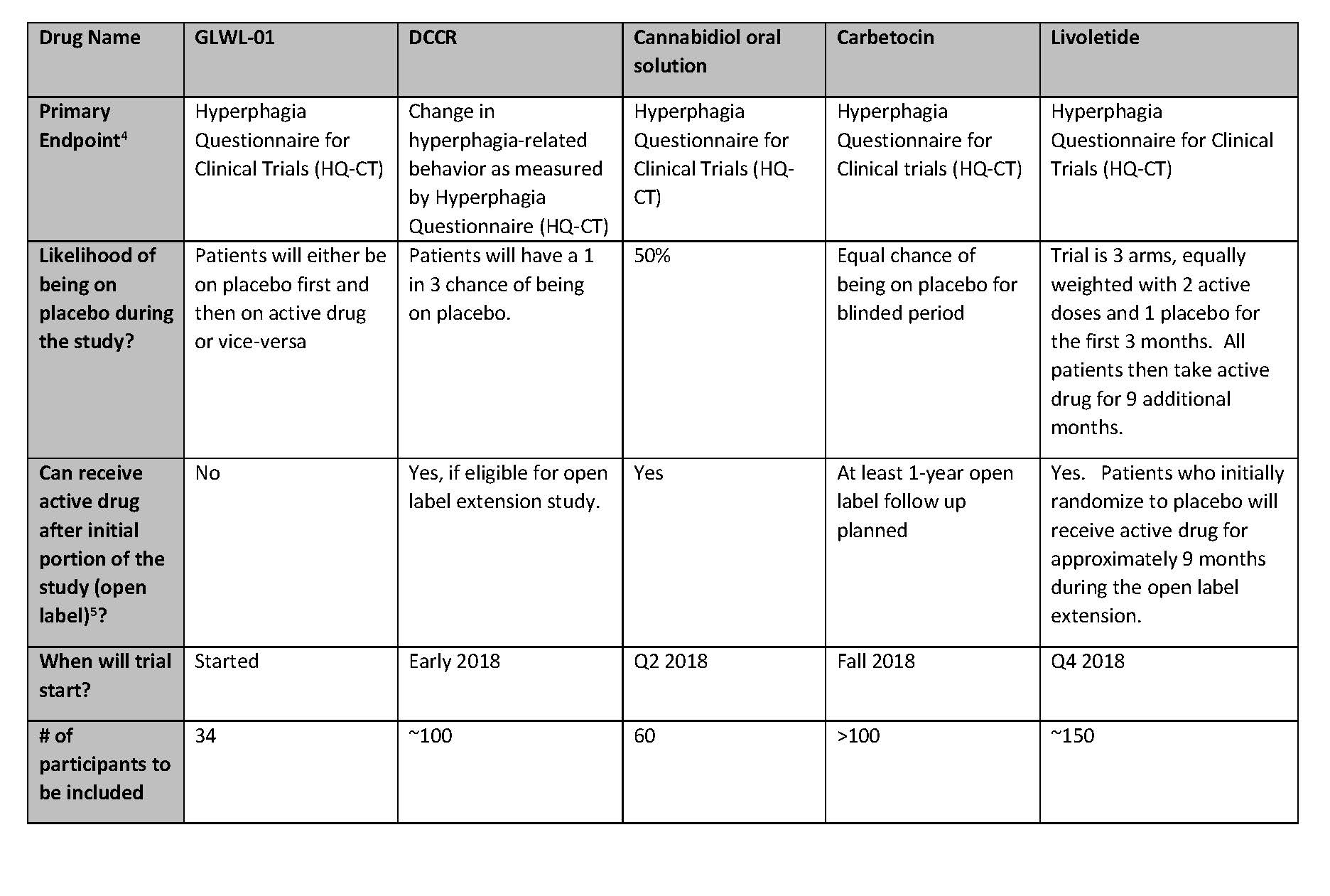 Summary of active clinical trials for prader willi syndrome
Summary of active clinical trials for prader willi syndrome
Clinical Trials. Frederick National Laboratory for Cancer Research . Bioinformatics, Big Data, and Cancer. Key Initiatives. Progress. Annual Report to the Nation. Research Advances by Cancer Type. Stories of Discovery. Milestones in Cancer Research and Discovery. Resources for Researchers. Cryo-EM.
Plos medicine safety and pharmacokinetics of the fc modified
The BRIGHT study is a Phase III 12-month, open-label, switch-over study designed to evaluate the safety, efficacy and pharmacokinetics of PRX–102 treatment, 2 mg/kg every four weeks, in up to 30 ...
 Xyloglucan for the treatment of acute diarrhea results of a
Xyloglucan for the treatment of acute diarrhea results of a
Open-label placebo treatment for cancer-related fatigue: a randomized-controlled clinical trial. Sci Rep (2018) 8(1):2784. doi: 10.1038/s41598-018-20993-y PubMed Abstract | CrossRef Full Text | Google Scholar
Imerge study myelodysplastic syndromes clinical trial
For example, it has been demonstrated that open-label placebos can improve symptoms in allergic rhinitis. However, the mechanisms of how placebos without concealment work remain unknown. Trial design: In order to examine expectancy effects we conducted a randomized controlled trial (N = 46), in which patients with allergic symptoms received either placebos without deception or no pills at all.
 Difference between clinical research and clinical data
Difference between clinical research and clinical data
An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India. Participants...
 An open label randomized controlled trial of low dose
An open label randomized controlled trial of low dose
At our state-of-the-art facilities, we design and print multi-language clinical labels for clients all over the world. We also utilise Interactive Response Technologies (IRT) to enable you to quickly create patient kits for any patient, in any country, without having to commit your clinical trial stock to a specific market based on uncertain ...
Plos one a single arm open label phase 2 clinical trial
Clinical trials into open-label placebos (OPL) appear to show promise for a range of self-reported conditions but have been hampered by small sample sizes and short duration. What are the new findings? Placebo concepts refer to: (1) 'methodological controls'; or (2) 'mind–body treatment interventions'. ...
Open label trials are sometimes referred to as "non-masked" or "unblinded." If the trial is a non-pharmacological study, such as a trial of devices, or psychological and physical treatments, it may be referred to simply as "open." After recruitment to the trial, the participants were allocated to treatment using block randomisation.
 An open label pragmatic randomized controlled clinical
An open label pragmatic randomized controlled clinical
In other words, "Open-label clinical trial" is a type of cohort study, and, in this case, "Open-label" is a more precise description of what is described in the stem. +5 . drdoom For a more technical explanation of "Cohort studies", see the definition from ...
32 what is an open label trial labels database 2020
New life-saving treatments for Malignant Solid Tumors in clinical trial on First-in-human open-label dose-escalation trial with expansion cohorts to evaluate safety of GEN1046 in subjects with malignant solid tumors
 Americanheartj on twitter rationale and design of proact xa
Americanheartj on twitter rationale and design of proact xa
Open-Label Trial A type of clinical trial. In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants.
 Debiopharm announces first patient dosed in investigator
Debiopharm announces first patient dosed in investigator
A multicenter, open-label trial to determine the safety of vismodegib, a Hedgehog pathway inhibitor, for treatment of advanced basal cell carcinoma is currently underway in 1227 patients (18 years and older) with local or metastatic advanced basal cell carcinoma and who are ineligible for surgery (NCT01367665).
 The clinical study design a singlecenter randomized open
The clinical study design a singlecenter randomized open
Clinical Trials Overview. Clinical trials are part of clinical research and at the heart of all medical advances. Clinical trials look at new ways to prevent, detect, or treat disease. Treatments might be new drugs or new combinations of drugs, new surgical procedures or devices, or new ways to use existing treatments.
 Potential impact of differential drop in of open label
Potential impact of differential drop in of open label
Protalix BioTherapeutics and Chiesi Global Rare Diseases Announce Positive Topline Results from BRIGHT Phase III Open-Label, Switch-Over Clinical Trial Evaluating Pegunigalsidase Alfa 2 mg/kg every Four Weeks for Treatment of Fabry Disease - read this article along with other careers information, tips and advice on BioSpace
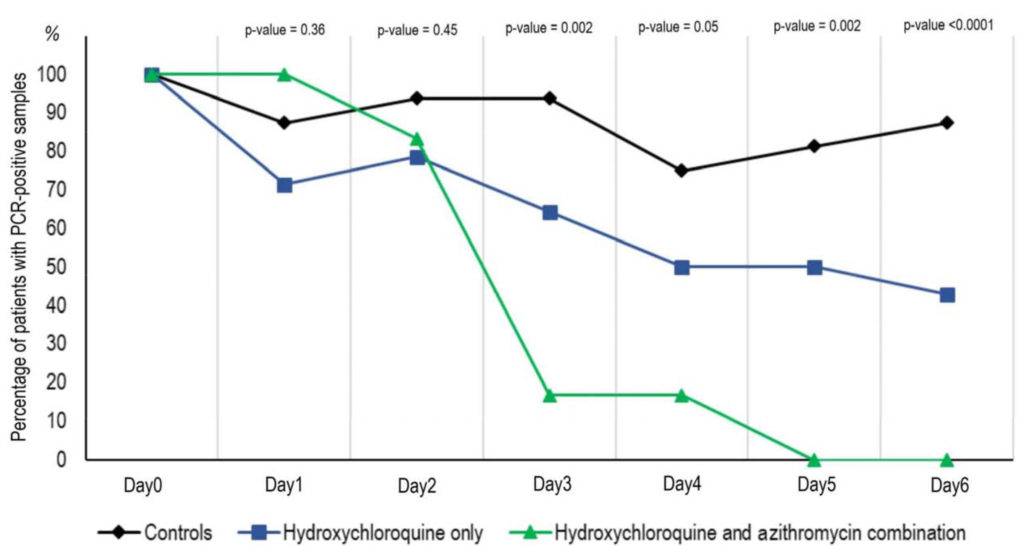 Hydroxychloroquine and azithromycin as a treatment of covid
Hydroxychloroquine and azithromycin as a treatment of covid
Open-label trials may be performed to study the effectiveness of similar medications. Before drugs can be sold and marketed in the U.S., they must undergo several safety and efficacy tests -- typically on animals and humans -- called clinical trials. Pharmacists may help develop clinical trials.
 A 51 week open label clinical trial in india to assess the
A 51 week open label clinical trial in india to assess the
Clinical trial for Myasthenia Gravis generalised | generalized myasthenia gravis | Myasthenia Gravis (Chronic Weakness) | Myasthenia Gravis , Open-Label Extension of Zilucoplan in Subjects With Generalized Myasthenia Gravis
Plos one efficacy and tolerability of rivastigmine patch
The Phase 1/2 open-label trial is designed to assess the safety and efficacy of a single dose of CTX001 in patients ages 18 to 35 with TDT, non-beta zero/beta zero subtypes. Vertex Pharmaceuticals (NASDAQ: VRTX) - CRISPR Therapeutics and Vertex Announce FDA Fast Track Designation for CTX001 for the Treatment of Beta Thalassemia -- 16/4/2019
 What is an open label trial with pictures
What is an open label trial with pictures
Confusing term, used to indicate that a clinical trial does not have any specific methodological characteristic. An open clinical trial is a clinical trial without a control group, as opposed to a controlled clinical trial. It can also be a non-blinded clinical trial, as opposed to a single-blind or double-blind clinical trial.
 Autologous bone marrow cell therapy for autism an open label
Autologous bone marrow cell therapy for autism an open label
To assess the adverse events of PVP-I TRIAL DESIGN: This is a single-center, open-label randomized clinical trial with a 7-arm parallel-group design. PARTICIPANTS: The study will be conducted at Dhaka Medical College Hospital, Dhaka, Bangladesh.
 The progress of clinical trials of psc derived cells and its
The progress of clinical trials of psc derived cells and its
The CD12 protocol will be amended for adding the open-label arm extension and submitted to the FDA on Monday, December 28, 2020. ... each CD12 participating clinical trial site will have the ...
0 Response to "23 Open-label Clinical Trial"
Post a Comment