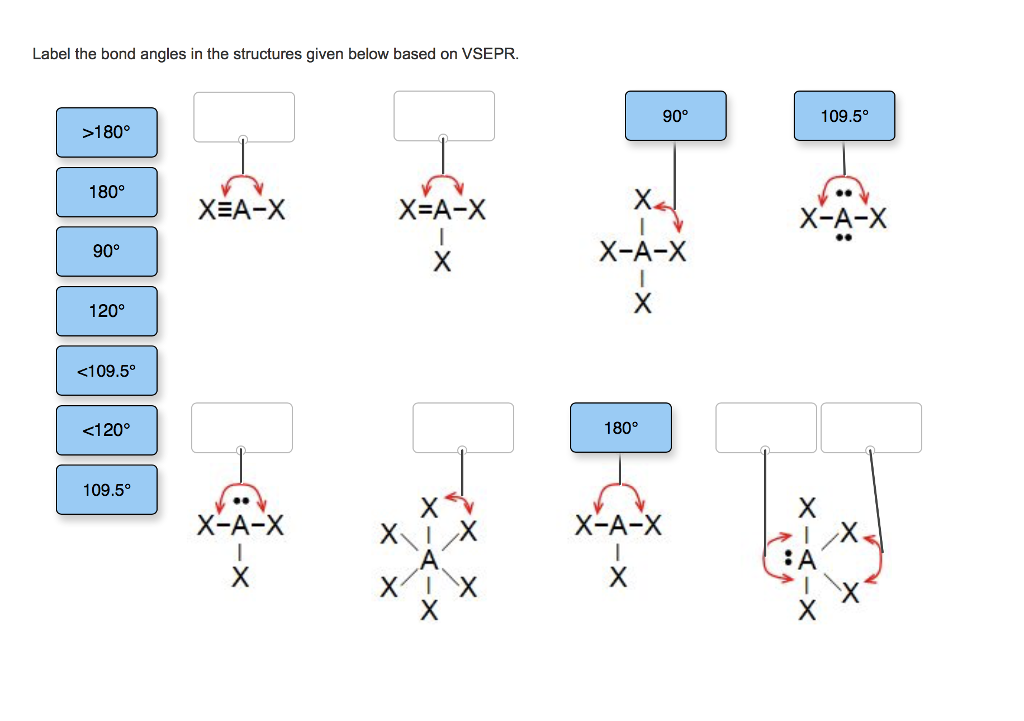
34 Label The Bond Angles In The Structures Given Below Based On Vsepr.
Ozone has angles of 107 degrees for both 7 109
Analyze We are given a Lewis structure and asked to determine two bond angles
 All bond angles in "AX"_2"E"_2 molecules are significantly less than 109 Bond angles will deviate
All bond angles in "AX"_2"E"_2 molecules are significantly less than 109 Bond angles will deviate
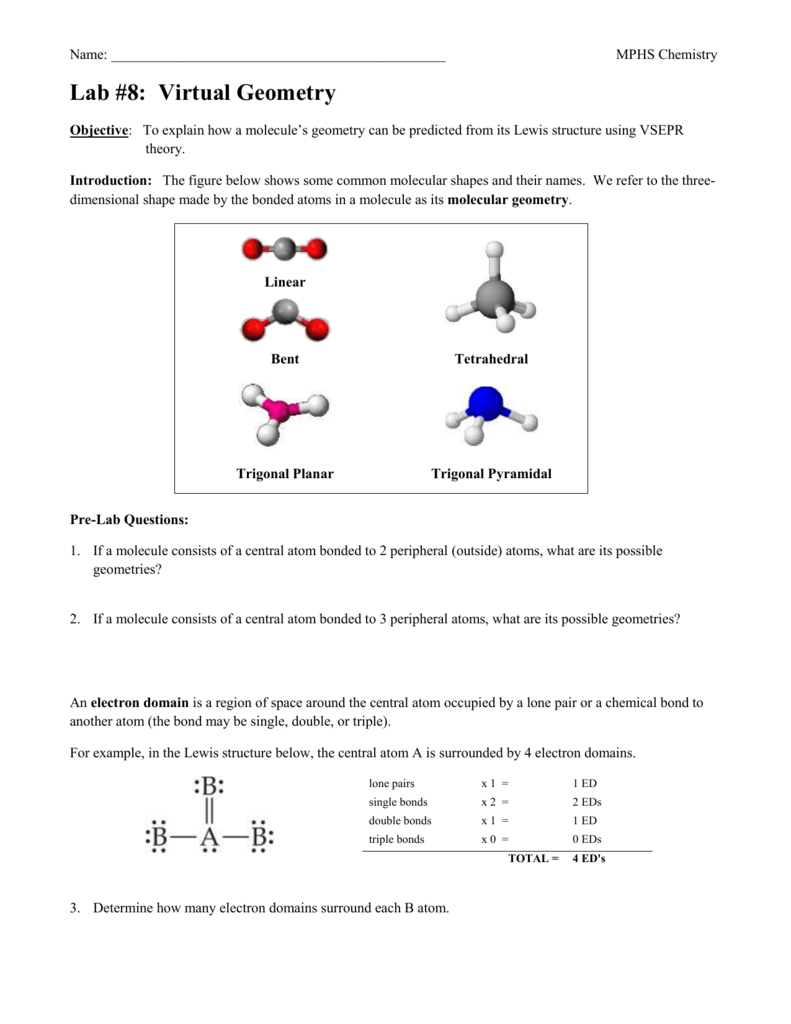
Label the bond angles in the structures given below based on vsepr.. The "H-O-H" bond angle is less than that in "NH"_3, partly because of the greater repulsions caused by two lone pairs 5 o
Using the simulation, determine the bond angles in CH 4, NH 3, and H 2 O for the Real vs For bent molecular geometry when the electron-pair geometry is tetrahedral the bond angle is around 105 degrees There is more than one bonding pair of electrons in a multiple bond but all of the electron pairs involved in a multiple bond must be in roughly the same place
We can draw the Lewis structure on a sheet of paper Solution Eyedrops for dry eyes usually contain a water-soluble polymer called poly(vinyl alcohol), which For the H—O—C bond angle, the middle O atom has four electron domains (two bonding and two nonbonding)
 Curved Arrows Assign Formal Charges Appropriate As B H Ch 153 Using Vsepr Course Hero
Curved Arrows Assign Formal Charges Appropriate As B H Ch 153 Using Vsepr Course Hero
 Molecular Geometry And Covalent Bonding Models
Molecular Geometry And Covalent Bonding Models
Lewis structures
Https Www Saddleback Edu Faculty Jzoval A Version2 0 Chapter 4 V 2 0 Ch4 Ppt Templates Chapter 4 Lecture Slides V2 Pdf
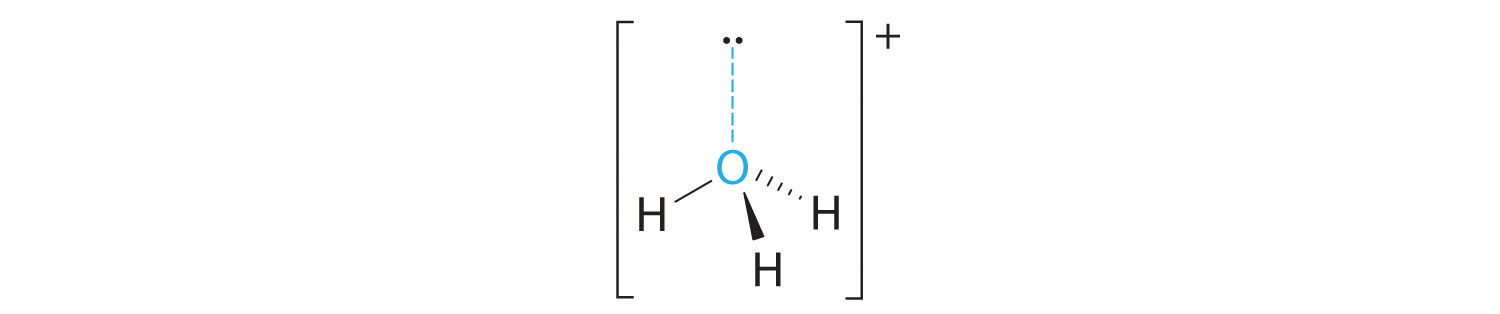
However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space ()
Hybrid Atomic Orbitals In Organic Chemistry Part 2 Critique Of Practical Aspects
5^@ in three dimensions, but the lone pair 'crunches' the atoms together a little, so the angles become less than 109
 Molecular Structure And Polarity Chem 1305 General Chemistry I Lecture
Molecular Structure And Polarity Chem 1305 General Chemistry I Lecture
 Ch9 Study Packet Flashcards Easy Notecards
Ch9 Study Packet Flashcards Easy Notecards
Measured bond angle VSEPR Idealized bond angle C-C-C 111
 Vsepr Theory Bond Angles Page 1 Line 17qq Com
Vsepr Theory Bond Angles Page 1 Line 17qq Com
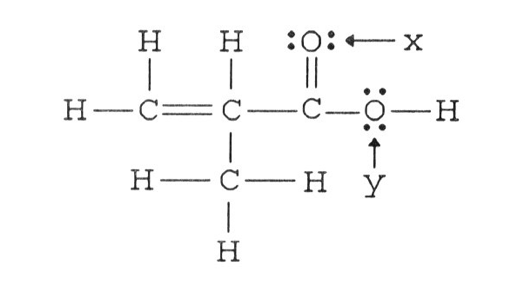
For example, in a molecule such as CH 2 O (AX 3), whose structure is shown below, the double bond repels the single bonds more strongly than the single bonds repel each other
 Quickly Determine The Sp3 Sp2 And Sp Hybridization Chemistry Steps
Quickly Determine The Sp3 Sp2 And Sp Hybridization Chemistry Steps
Which has the least bond angle [NCERT 1973; DPMT 1990; CBSE PMT 1990; UPSEAT 2003]
 Solution Label The Bond Angles In The Str Clutch Prep
Solution Label The Bond Angles In The Str Clutch Prep
yramidal Geometry with 120° and 90° bond angles 3 groups are in a single plane (Equatorial Positions) resembling the trigonal planar geometry with 120° bond angles, and 2 groups are above and below the planes (Axial Positions) with 90° bond angles to the trigonal plane The valence shell electron pair repulsion (VSEPR) model is a tool of the valence bond method; it is based on
Http Chemistrye Weebly Com Uploads 3 7 7 0 37707825 U1ws6 Bonds Vsepr Shapes Hybrid Pdf
Indeed, the first two molecules have smaller bond angles, as expected, but the bond angle in OCl 2 is 110
 Chemistry By Sanitationambasador Issuu
Chemistry By Sanitationambasador Issuu
Version 1
 Shown Below Is The Partial Structural Formula For The Amino Acid L Cysteine Hsch2 Nh2 Chcooh Th Homeworklib
Shown Below Is The Partial Structural Formula For The Amino Acid L Cysteine Hsch2 Nh2 Chcooh Th Homeworklib
The VSEPR model assumes that electron pairs in the valence shell of a central atom will adopt an arrangement that minimizes repulsions between these electron pairs by maximizing the distance between them
 Molecular Structure And Polarity Chem 1305 General Chemistry I Lecture
Molecular Structure And Polarity Chem 1305 General Chemistry I Lecture
The Lewis structure of carbon dioxide is given below
 Solved Please Give Me The Process For Solving This Problem Chegg Com
Solved Please Give Me The Process For Solving This Problem Chegg Com
and it relates molecular geometry to the atomic
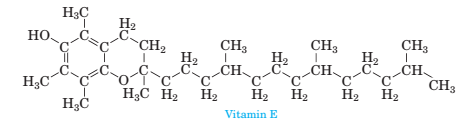 3 105 Consider The Structure Of Vitamin E Shown Below Which Is Found Most Abundantly In Wheat Germ Oil Sunflower And Safflower Oils A Identify The Various Types Of Geometries Present In Each
3 105 Consider The Structure Of Vitamin E Shown Below Which Is Found Most Abundantly In Wheat Germ Oil Sunflower And Safflower Oils A Identify The Various Types Of Geometries Present In Each
9°, more than a degree larger than the tetrahedral angle
Https Www Ddtwo Org Site Handlers Filedownload Ashx Moduleinstanceid 35942 Dataid 51186 Filename Covalent 20bonding 20lecture 20slides Pdf
SN = 5 There are four possibilities
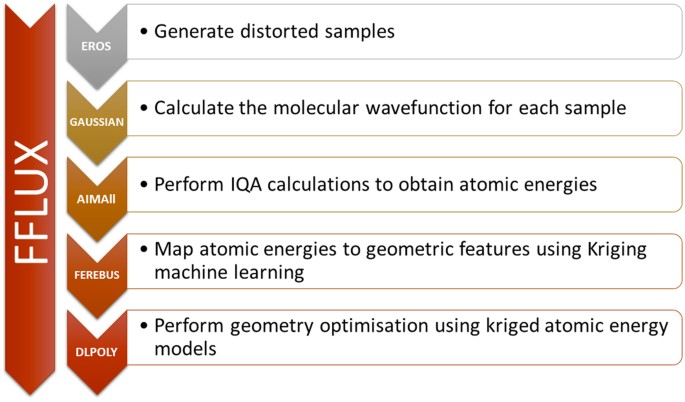 Geometry Optimization With Machine Trained Topological Atoms Scientific Reports
Geometry Optimization With Machine Trained Topological Atoms Scientific Reports
valence shell electron-pair repulsion theory (VSEPR) theory used to predict the bond angles in a molecule based on positioning regions of high electron density as far apart as possible to minimize electrostatic repulsion vector quantity having magnitude and direction Honors Chemistry Name: _____ Period: ___ Date: ___ VSEPR Molecular Models Lab Objective: To predict structure and polarity of a molecule based on its Lewis Dot structure and VSEPR Theory
 34 Label The Bond Angles In The Structures Given Below Based On Vsepr Labels Design Ideas 2020
34 Label The Bond Angles In The Structures Given Below Based On Vsepr Labels Design Ideas 2020
VSEPR model helps to understand the different shapes and arrangement of molecules
Http Seaver Faculty Pepperdine Edu Jfritsch 121 20web Ch 2010 20thru 2011 20practice 20problems 20key 20012717 Pdf
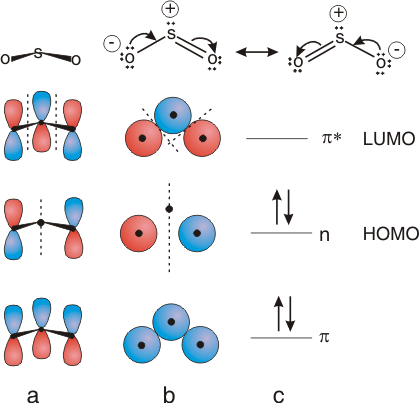
VSEPR Theory - Multiple bonds
 34 Label The Bond Angles In The Structures Given Below Based On Vsepr Labels Design Ideas 2020
34 Label The Bond Angles In The Structures Given Below Based On Vsepr Labels Design Ideas 2020
We have claimed that the two lone pairs on the O atom (not shown) should push the bonding pairs of electrons down, lowering the bond angle from the perfect tetrahedral angle of 109
 Vsepr For 4 Electron Clouds Video Vsepr Khan Academy
Vsepr For 4 Electron Clouds Video Vsepr Khan Academy
Label the bond angles in the structures given below based on VSEPR
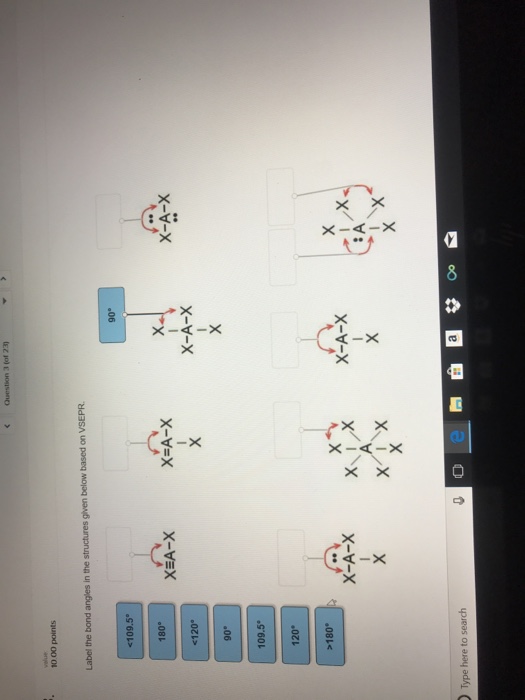
A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees
 30 Label The Bond Angles In The Structures Given Below Based On Vsepr Labels For Your Ideas
30 Label The Bond Angles In The Structures Given Below Based On Vsepr Labels For Your Ideas
The electron-domain geometry around O is therefore tetrahedral, which gives an ideal angle of 109
A Level Gce Shapes Of Molecules Appendix Shapes Of Oxyanions Ks5 As A2 Revision Notes
According to VSEPR theory, the most probable shape of the molecule having 4 electron pairs in the outer shell of the central atom is [MP PET 1996, 2001]
Hybrid Atomic Orbitals In Organic Chemistry Part 2 Critique Of Practical Aspects
1-6
 Ch105 Chapter 5 Introduction To Organic Chemistry Chemistry
Ch105 Chapter 5 Introduction To Organic Chemistry Chemistry
CHM151LL: VSEPR and Molecular Geometry Tables © GCC, 2006 page 2 of 6 4 Molecular Geometries Where Central Atom Has No Lone Pairs # of Outer Atoms General Formula Molecular Geometry and Bond Angles Name 2 AB2 linear 3 AB3 trigonal planar 4 AB tetrahedral 5 AB5 trigonal bipyramidal 6 AB6 octahedral 180 120 120° 90° 90° 109
0 Response to "34 Label The Bond Angles In The Structures Given Below Based On Vsepr."
Post a Comment