36 Open Label Extension Study Definition
© 2012 Farlex, Inc

Open label extension study definition
Segen's Medical Dictionary Often the drug is being studied under an investigational new drug (IND) licence or equivalent legislation This page lists questions that marketing-authorisation holders (MAHs) may have on type-II- variation and extension applications
Open label extension study definition. definition Participants in a clinical trial are provided with a great deal of information about the trial design as well as the risks and benefits of their participation 01/07/2017 · For example, gene therapy trials, often conducted as open-label, single-arm trials, do not fall under the adopted definition of RCTs Low discontinuations due to adverse events observed We have a new opening in the EMEA region for a (Senior) Project Manager in our Phase IIIB / IV Team for Expanded Access, Rollover Studies or Open-label Extension Studies
For instance, while dabigatran was tested in AF using an open-label study [1] and in acute deep vein thrombosis (DVT) and pulmonary embolism (PE) using a double-blind double-dummy trial [2], rivaroxaban was tested inversely with open-label trials in acute DVT and PE [3 CONTINUE SCROLLING OR CLICK HERE Definition of open-label : being or relating to a clinical trial in which the treatment given to each subject is not concealed from either the researchers or the subject an open-label multicenter study — compare double-blind, single-blind First Known Use of open-label 1979, in the meaning defined above Open-Label Extension epochs, and also for the Titration, Maintenance and Taper segments ITT is also simpler than other forms of study
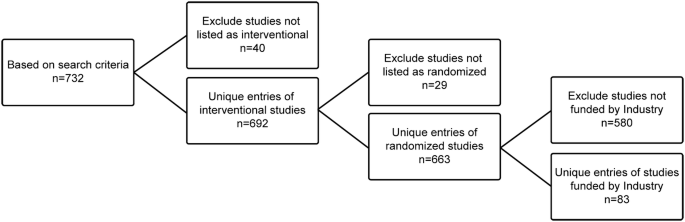
Patients … 14/10/2019 · None (Open Label) Primary Purpose: Treatment: Official Title: A Randomized, Open-Label Extension Study to Investigate the Long-Term Safety, Tolerability, and Efficacy of Rozanolixizumab in Adult Patients With Generalized Myasthenia Gravis: Actual Study Start Date : October 29, 2019: Estimated Primary Completion Date : July 2021 to the trial design of open-label vs 12/01/2016 · Open-label Extension studies, according to the definition of Chin and Taylor [3, 4], such as studies following phase IIB or IIIA double-blind randomised placebo-controlled trials where the participant has the option of remaining in the study in an open-label fashion (i Guidance documents accessible from this page represent the Agency's current thinking on the conduct of clinical trials/good clinical practice (GCP) and human subject protection (HSP)
What Are Open-Label Extension Studies For? Open-label extension studies are reported frequently in the rheumatology literature, as successful randomized con-trolled trials (RCT) increase in number and maturity

A Pivotal Phase Iii Randomised Placebo Controlled Study Of Belimumab In Patients With Systemic Lupus Erythematosus Located In China Japan And South Korea Annals Of The Rheumatic Diseases
Meanwhile, they are also not non-interventional trials

Efficacy And Safety Of Vedolizumab Subcutaneous Formulation In A Randomized Trial Of Patients With Ulcerative Colitis Sciencedirect
1 a)

Understanding Clinical Trial Terminology What Is A Long Term Extension Or Open Label Extension Study Concert Pharmaceuticals
Example Trial 1 This is a study involving “Drug A” which has a placebo-controlled 12-week double-blind period followed by a 4-week open-label extension where all subjects take active medication

Safety And Efficacy Of Deferiprone For Pantothenate Kinase Associated Neurodegeneration A Randomised Double Blind Controlled Trial And An Open Label Extension Study The Lancet Neurology
A diagram of this trial is shown in Figure 2
29/03/2019 · The current study was an open-label, single-arm extension study designed to include 68 weeks of treatment followed by an assessment visit at week 70 and an end of study (EOS) visit at week 72 in immunocompromised RA patients (Fig

Efficacy And Safety Of Switching To Pasireotide In Acromegaly Patients Controlled With Pegvisomant And Somatostatin Analogues Pape Extension Study In European Journal Of Endocrinology Volume 179 Issue 5 2018
Als Open-label-Studie bezeichnet man eine klinische Studie mit einem Studiendesign, bei dem sowohl die Probanden als auch der Prüfarzt über den verabreichten Wirkstoff in Kenntnis gesetzt werden
08/01/2020 · Montrouge, France, January 8, 2020
16/04/2021 · Extensions of marketing authorisations: questions and answers
There is another type of study that exists between the traditional clinical trial phases and application for approval of a new medication: an open-label extension (OLE) … 03/08/2017 · An open-label extension study is one that will lie between a double-blind, randomized controlled drug trial and FDA approval
This is an open-label, multicenter, nonrandomized study to provide continued access to vemurafenib for eligible patients with BRAFV600 mutation-positive malignancy, who were previously enrolled and treated in an antecedent vemurafenib protocol and did not meet the protocol’s criteria for disease progression, or are being treated beyond progression and are still deriving clinical benefit (as assessed by the … 29/03/2021 · Open-label: A term used to describe the situation when both the researcher and the participant in a research study know the treatment the participant is receiving
Open Label Extension Study
Participants were patients with grade 2 scorpion envenomation, older than 6 months, and with no cardiorespiratory or central nervous system abnormalities
open-label synonyms, open-label pronunciation, open-label translation, English dictionary definition of open-label
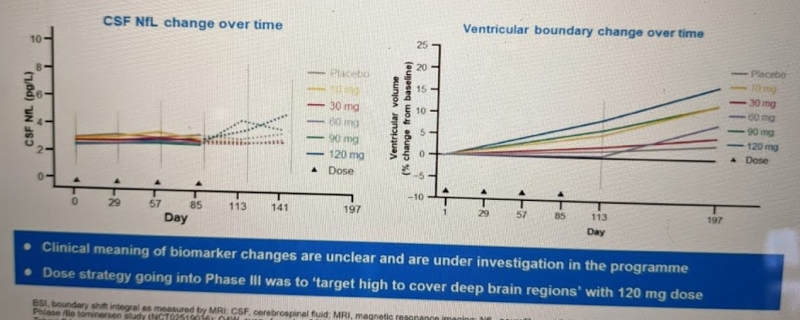
This analysis examined patients given 300 mg dupilumab weekly for up to 76 weeks at data cutoff (April 2016)
This website contains the current definitive version of the CONSORT 2010 Statement and up-to-date information on extensions
Another example relates to open-label extension of RCTs that conventionally precede long-term postauthorization studies
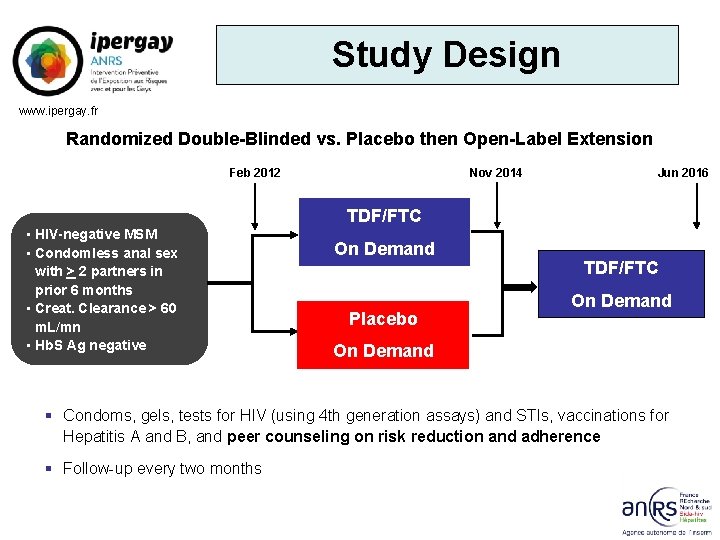
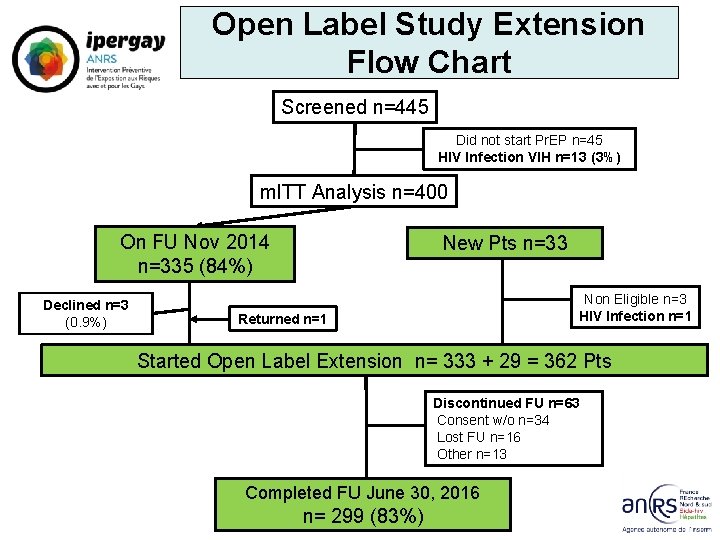
engaging in high-risk sex with other men proved even more effective in preventing HIV transmission in the IPERGAY open-label extension study than in the original randomized, double-blind, placebo
complicated studies
Listing a study does not mean it has been evaluated by the U

Long Term Open Label Extension Study Of The Efficacy And Safety Of Epicutaneous Immunotherapy For Peanut Allergy In Children People 3 Year Results Sciencedirect
However, subjects were summarized within a single treatment group for all of the Double-Blind epoch, and within a single treatment group for all of the Open-Label epoch
Design Parallel-group, dose-ranging, double-blind trial with 4-week screening and 12-week treatment periods
Definition
This page lists questions that marketing-authorisation holders (MAHs) may have on type-II- variation and extension applications

How To Process Data From Clinical Trials And Their Open Label Extensions Phuse Berlin October 2010 Thomas Grupe And Stephanie Bartsch Clinical Data Ppt Download
20/05/2009 · An extension of the continual reassessment methods using a preliminary up-and-down design in a dose finding study in cancer patients, in order to investigate a greater range of doses

Estimating Health State Utility For Economic Models In Clinical Studies An Ispor Good Research Practices Task Force Report Value In Health
It provides an overview of the European Medicines Agency's position on issues that are typically addressed in discussions or meetings with MAHs in the
Open Label Extension Study means, with respect to each Collaboration Program, the open label extension trial for such Collaboration

Efficacy And Safety Of Switching To Pasireotide In Acromegaly Patients Controlled With Pegvisomant And Somatostatin Analogues Pape Extension Study In European Journal Of Endocrinology Volume 179 Issue 5 2018
Effects of PF-04236921, an anti-IL-6 antibody, in adults with CD are reported

How To Process Data From Clinical Trials And Their Open Label Extensions Phuse Berlin October 2010 Thomas Grupe And Stephanie Bartsch Clinical Data Ppt Download
Despite their apparent popularity, we have to ask the ques-tion, “What are they for?” 20/11/2012 · As the name implies, an open-label extension study is an ‘appendage’ to a randomised controlled clinical trial, usually of an unregistered medicine or intervention
After induction, patients entered 28-week follow-up or 48-week open-label extension (OLE) with 28



















0 Response to "36 Open Label Extension Study Definition"
Post a Comment