34 Which Of The Following Is Not A Requirement For A Container Label?
The labels do not include the manufacturer's name and address, nor does the label have a hazard statement. Question 1: Does the pre-printed labeling on these bottles suffice for labeling secondary containers in the workplace under 29 CFR 1910.1200(f)(6)(ii)? Response: Yes. Section 1910.1200(f)(6)(ii) requires that workplace labeling include. Prior to shipping the containers from the on-site warehouse, an HCS 2012 label, compliant with paragraph 1910.1200(f)(1), is required on the outside of the shipping container or attached to any accompanying shipping papers or bill of lading.
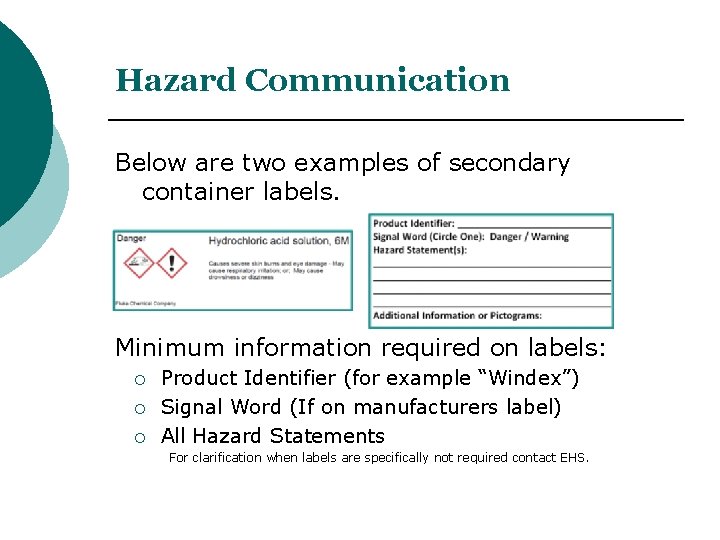
OSHA Requirements for Secondary Container Labels. OSHA requires secondary container labels to have the full GHS label, or: "Product identifier and words, pictures, symbols, or combination thereof, which provide at least general information regarding the hazards of the chemicals, and which, in conjunction with the other information immediately.

Which of the following is not a requirement for a container label?
c-The medication lot number does not need to appear on the prescription label. The required information on a prescription label includes the date when the prescription was filled, serial (prescription) number of the prescription, pharmacy name and address, patient name, prescribing physician name, all directions for use of the prescription, medication strength, drug manufacturer name, drug. The HCS 2012 Shipped - Primary Container Label Requirements. Under the new HSC 2012, labels on primary containers shipped from manufacturers or distributors, the container must be labeled, tagged or marked with the following six items: Product Identifier . A product identifier means the name or number used for a hazardous product on a label or. In this article. Microsoft 365 licensing guidance for security & compliance.. In addition to using sensitivity labels to classify and protect documents and emails, you can also use sensitivity labels to protect content in the following containers: Microsoft Teams sites, Microsoft 365 groups (formerly Office 365 groups), and SharePoint sites.For this container-level classification and.
Which of the following is not a requirement for a container label?. There is no HCS requirement that a container label must look like the example provided above. The only requirement is that all of the required information be on the label. 1 A container means any bag, barrel, bottle, box, can, cylinder, drum, reaction vessel, storage tank, or the like that contains a hazardous chemical. For Assessors c-The medication lot number does not need to appear on the prescription label. The required information on a prescription label includes the date when the prescription was filled, serial (prescription) number of the prescription, pharmacy name and address, patient name, prescribing physician name, all directions for use of the prescription, medication strength, drug manufacturer name, drug. However, FALCPA's labeling requirements do not apply to foods that are placed in a wrapper or container in response to a consumer's order - such as the paper or box used to provide a sandwich. Which of the following is a violation of the OSHA standard with respect to biohazard sharps containers? a. Locate the sharps container as close as possible to the area of use. b. Maintain sharps containers in an upright position. c. Only reach into a sharps container with a gloved hand. d. Replace sharps containers when they are 3/4 full.
If the shipping container is the actual container holding the hazardous chemical, it would have to be labeled in accordance with the HCS, but labeled in such a way that the "appropriate hazard warning" did not interfere with any DOT required shipping labels or container shipping information. We hope this clarifies this issue for you. (iv) The transport vehicle or freight container contains no other material, hazardous or otherwise; and (v) The identification number marking requirement of this paragraph (a)(3) does not apply to Class 1, Class 7, or to non-bulk packagings for which identification numbers are not required. (b) Technical names. Which of the following is not required on a prescription label? A. The name and address of the pharmacy B. The physician's DEA number for controlled substances C. The patient's name D. The expiration date of the medication What volume must be entered in the computer system? 480 mL. Which of the following is not required on a prescription? Patient's social security number. What is the maximum number of refills permitted on a schedule IV prescription? 6. What is the purpose of an auxiliary label?
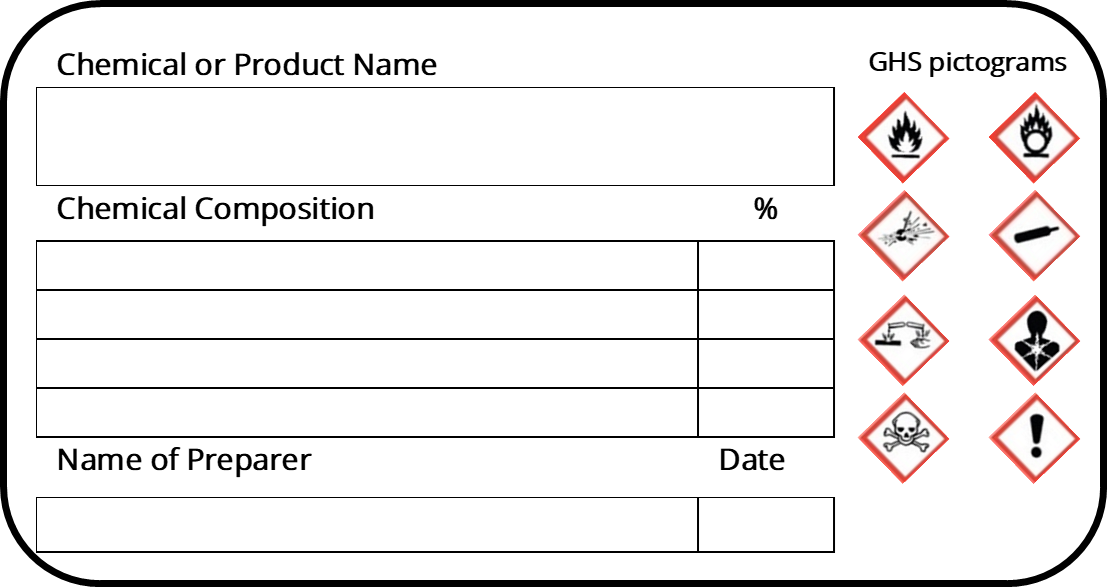
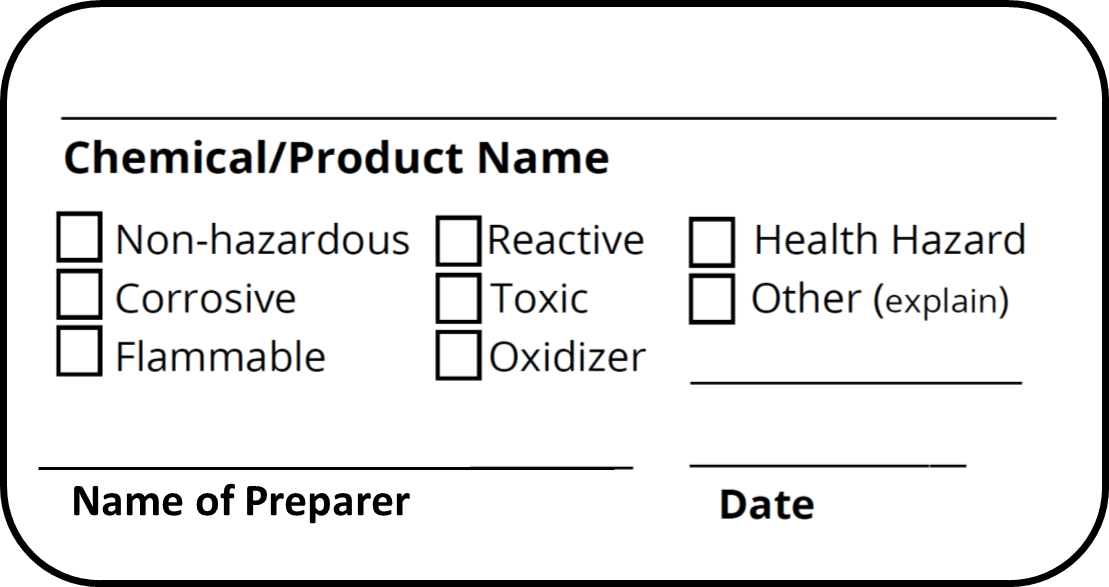
Labeling Requirements for Secondary Containers. These secondary containers are required to be labeled with a GHS chemical label, given if any of the following events occur: -The material is not used within the work shift of the individual who makes the transfer. -The worker who made the transfer leaves the work area. In this article. Microsoft 365 licensing guidance for security & compliance.. In addition to using sensitivity labels to classify and protect documents and emails, you can also use sensitivity labels to protect content in the following containers: Microsoft Teams sites, Microsoft 365 groups (formerly Office 365 groups), and SharePoint sites.For this container-level classification and. A labeled container is not a replacement for having the appropriate SDS available to the employee. Used drink or food containers are not to be used for secondary containers since the chemical may react with the leftover residue, or employees may think what is on the label is what is in the container (i.e. sports drink) and could accidentally. Sample GHS Container Label. As you can see, the GHS shipped/primary container label below provides much more information than the older HCS primary container label shown in Section 2. This label is intended to be an immediate visual reminder of the hazards of a chemical. However, it isn't necessary to list every hazard of the chemical on the label.
(d) The symbol is not required on a carton or wrapper in which a commercial container is held if the symbol is easily legible through such carton or wrapper. (e) The symbol is not required on a commercial container too small or otherwise unable to accommodate a label, if the symbol is printed on the box or package from which the commercial.
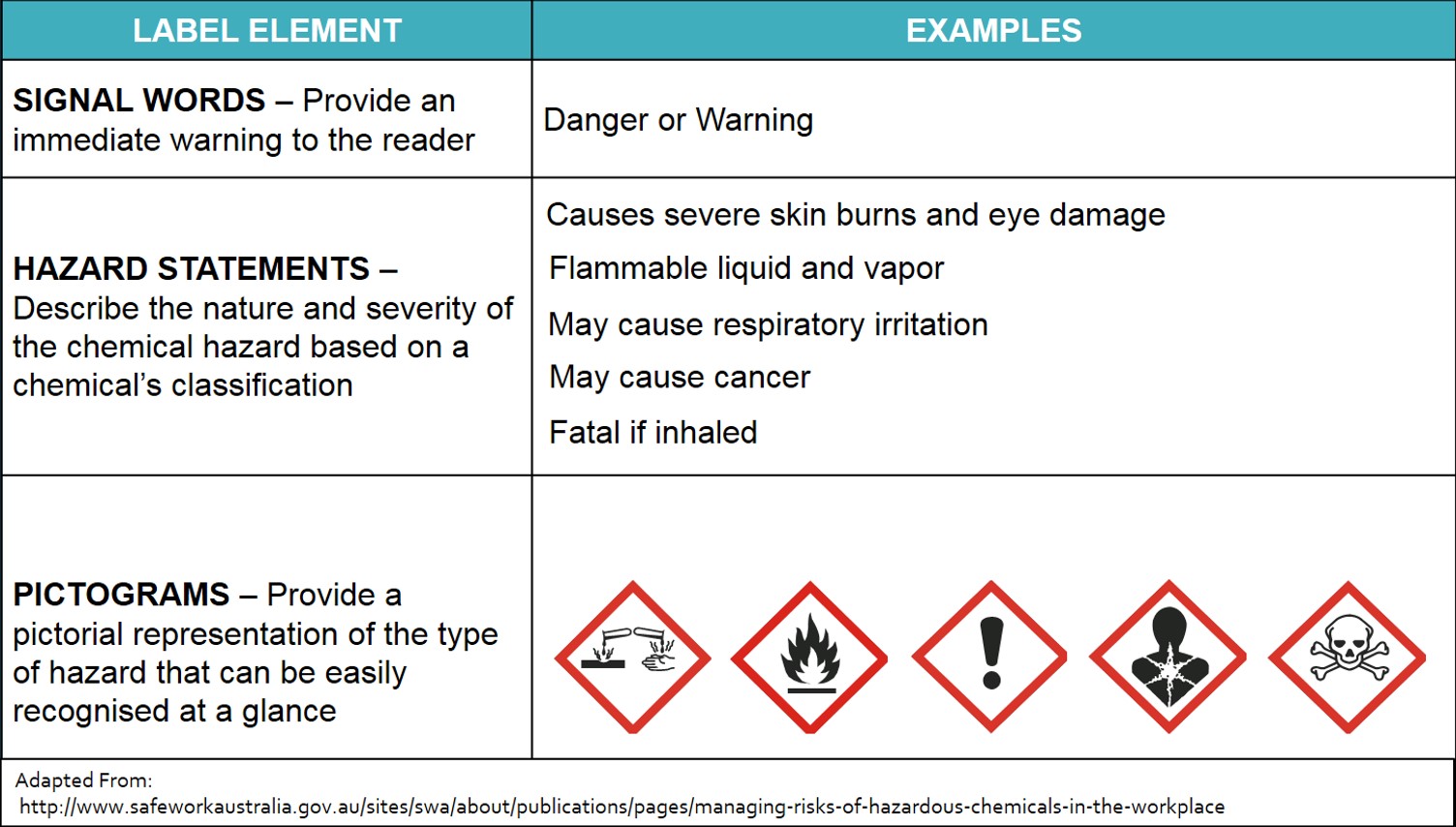
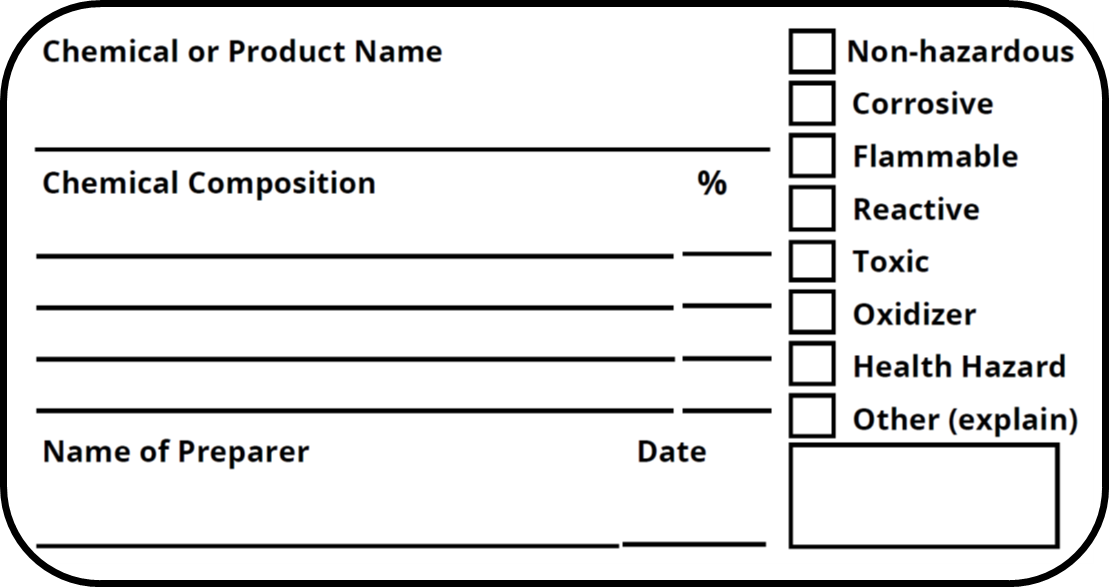
The 6 Main Elements of a GHS Label GHS-compliant labels contain six main elements. Note that these requirements apply to primary containers (which includes the containers received from the manufacturer), but not specifically to secondary containers (such as smaller jars or spray bottles that hold chemicals transferred from the primary container).
Required for peroxide formers, reactive substances; etc. Required for peroxide formers, reactive 1 This information may be added to the secondary container with an indelible pen, commercially available label, or by printing a label via the EH&S Assistant database. 2 The container produced by the chemical manufacturer that is delivered to the.
Secondary container labels are not required if both of the following apply: The reagent, stock solution and chemicals mixed for use are under the direct control of the person who transferred or prepared it, and; The container will be emptied during that person's work shift. Secondary chemical container label templates. Note: New label.
The HCS 2012 Shipped - Primary Container Label Requirements. Under the new HSC 2012, labels on primary containers shipped from manufacturers or distributors, the container must be labeled, tagged or marked with the following six items: Product Identifier . A product identifier means the name or number used for a hazardous product on a label or.
Under the HCS, the manufacturer, importer, or distributor is required to label each container of hazardous chemicals. Therefore, each container of hazardous chemicals received should have existing labels that comply with the requirements of the rule, and your clients would not be required to relabel the containers, shelves or doors.
Every specimen brought to the laboratory must have a label on the container in which it is held. It is not acceptable to label only the lid, transport bag, or other container used to transport the specimen. The label must contain the following legible information: Patient name; Patient medical record number, with check digit; Patient location
The outer container does not require a WHMIS label if the label on the inner container is visible and legible through the outer container under normal conditions of storage and handling, or The outer container has a label that meets the requirements set out in the Transportation of Dangerous Goods Regulations.
The master label bears a statement that the secondary container must be labeled as presented on the master label (e.g., “When this product is diluted in accordance with the directions on this label, the dilution container must bear the following statements:”) The secondary container contains a statement prohibiting further sale or distribution.
Container Label Size. 25 the following: 26... 82 requirements as they relate to the prevention of medication errors, product sponsors should refer
Containers also must have the following identification on the container, label or tag [29 CFR 1910.1200(f)(1)]: The identity of the hazardous chemical Appropriate hazard warnings
Again, if the incoming container is already labeled by the chemical manufacturer, importer, or other responsible party, then the employer is not required to affix his own label. If the container does require labeling by the employer, then either the alternative labeling method or a label containing the chemical identity and an appropriate.
OSHA requires every original chemical container to have a primary shipping label from the manufacturer with a few exceptions: Drugs for patient care, consumer chemicals, and pesticides, including disinfectants and dental unit waterline cleaners, are not subject to the labeling requirements. The chemical manufacturer must ensure that primary label is marked with the following information:
Except for a few cases, secondary containers must be labeled. IF IN DOUBT, LABEL IT! One common case where you do not have to label a secondary container is if the container is portable and will be used immediately by the person who transferred the chemical into that container.
Very small final container labels are exempted from this requirement. (9) The following information shall appear on the final container label and carton label, if any, but need not appear on the enclosure: (i) A permitted expiration date; (ii) The number of doses where applicable;

















0 Response to "34 Which Of The Following Is Not A Requirement For A Container Label?"
Post a Comment