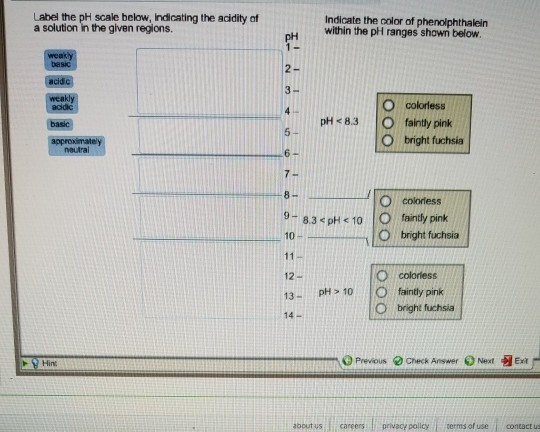
40 Label The Ph Scale Below, Indicating The Acidity Of A Solution In The Given Regions.
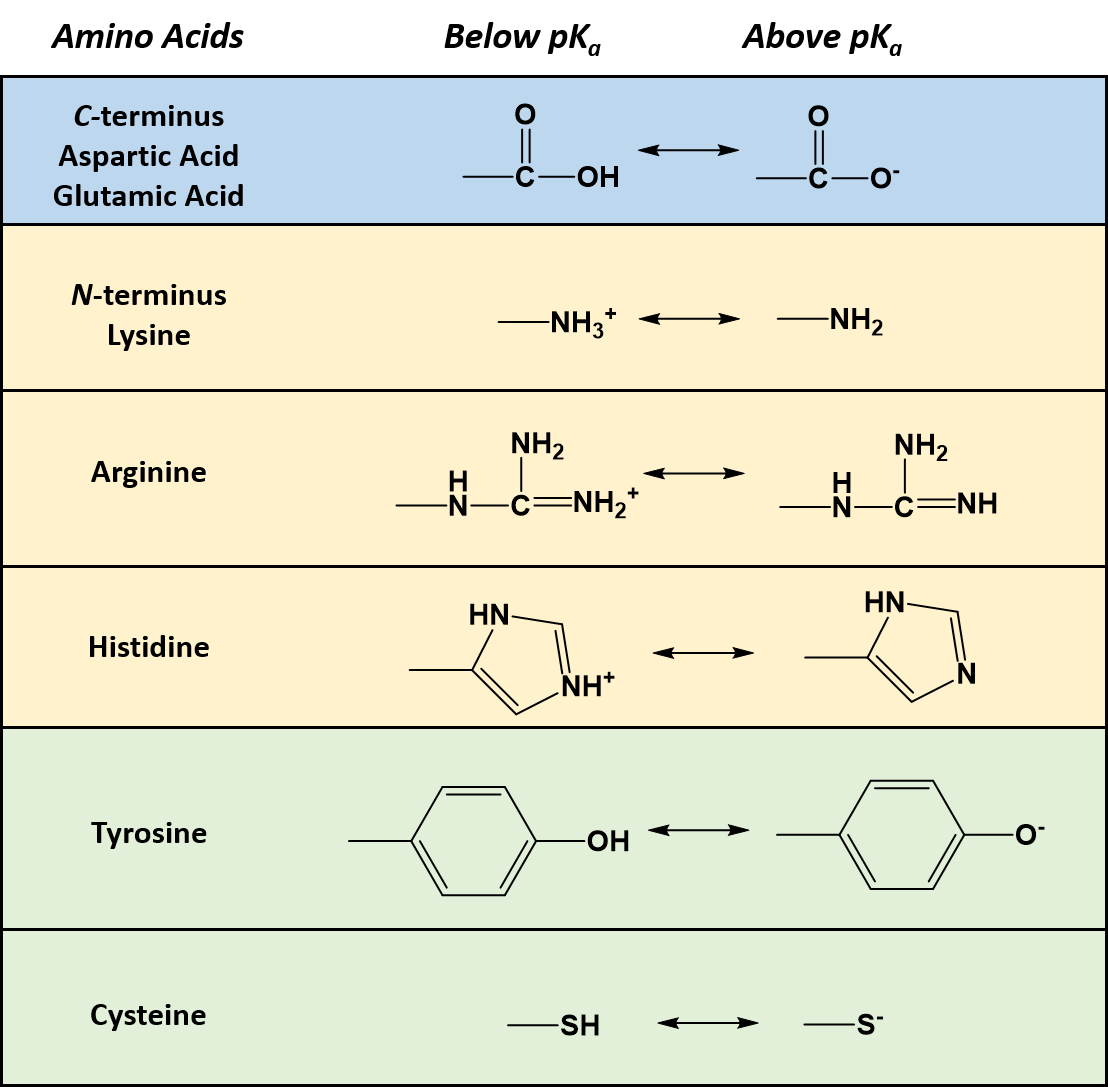
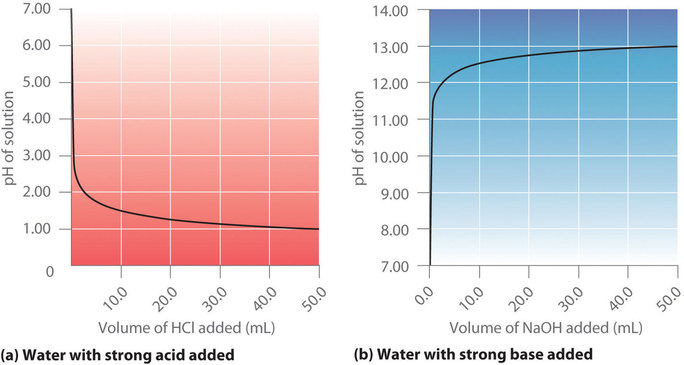
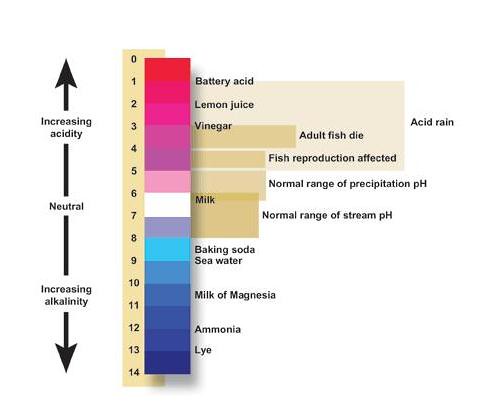
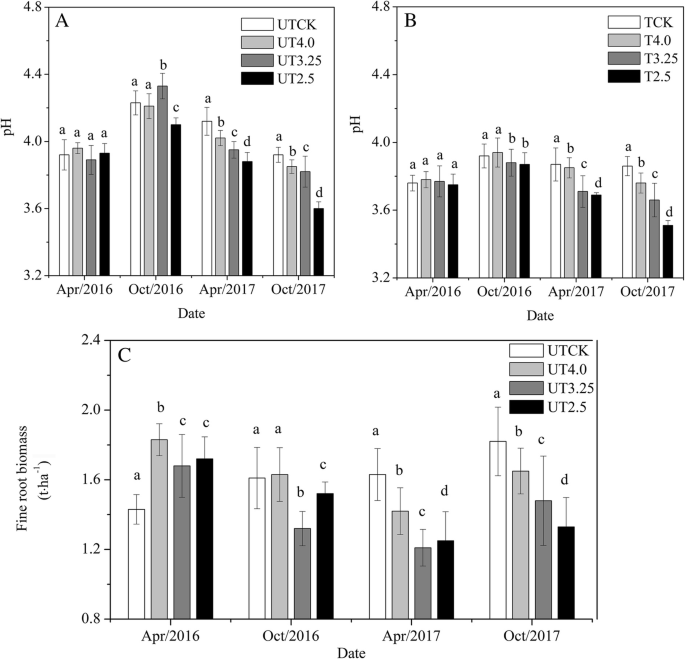
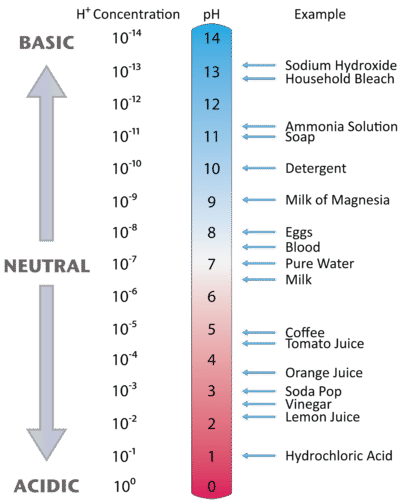
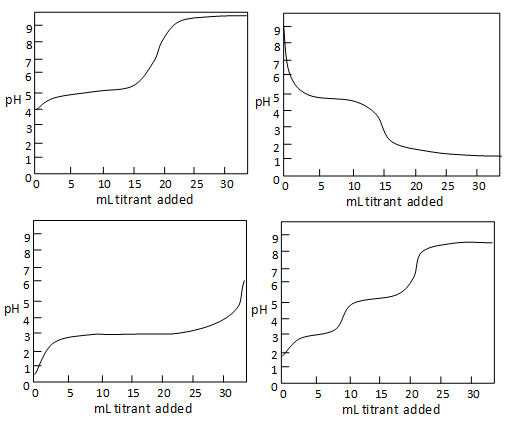
A The equivalence point of the titration and the phenolphthalein end point differ by less than one pH unit so Question Label the pH scale below indicating the acidity of a solution in the given regions Indicate the color of phenolphthalein within the pH ranges shown below Answer to Label the pH scale below indicating the acidity of a Skip to main content indicating the acidity of a solution in the given regions Indicate the color of phenolphthalein within the pH ranges shown below basic 2 3 4 approximately neutral O colorless O faintly pink O bright fuchsia weakly basic pH 83 weakly acidic acidic 6 7 8 Detailed Description pH is a measure of how acidicbasic water is The range goes from 0 - 14 with 7 being neutral pHs of less than 7 indicate acidity whereas a pH of greater than 7 indicates a base pH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water The pH scale The pH scale is used to rank solutions in terms of acidity or basicity alkalinity Since the scale is based on pH values it is logarithmic meaning that a change of 1 pH unit corresponds to a ten-fold change in H ion concentration The pH scale is often said to range from 0 to 14 and most solutions do fall within this range pH Scale a scale used to define the levels of hydrogen H or hydroxide OH ions in a solution Ranges from 0 acidic to 14 basicalkaline with 7 being neutral Label the pH scale below indicating the acidity of a solution in the given regions Indicate the color of phenolphthalein within the pH ranges shown below pH basic 2 3 4 approximately neutral weakly basic O colorless pH 83 weakly acidic faintly pink O bright fuchsia acidic 6 O colorless 9- 83 pH 1 faintly O bright fuchsia O colorless O bright fuchsia 12- pH10O faintly pink The pH scale ranges from 0 to 14 The pH of a solution is a measure of its acidity or alkalinity base You have probably used litmus paper paper that has been treated with a natural water-soluble dye so it can be used as a pH indicator to test how much acid or base alkalinity exists in a solution Increases Acidity 1 Adding 1M H- to a solution 2 Decreasing pH Decreases Acidity 1 Adding 1M -OH to a solution 2 Removing H-3 Adding base 4 Removing acid 5 Adding 1M H- to a solution while simultaneously adding 2M -OH The pH Scale Acidic Neutral and Basic The pH scale describes the acidity of the solution acidic neutral or basic A solution with a pH less than 7 is an acid exactly 7 is a neutral solution and above 7 is a base Bases have less hydrogen ions but more hydroxide ions represented by the pOH or “potential of hydroxide ions” Table 1 LAbel the pH scale below indicating the acidity of a solution in the given regions Indicate the color of phenoiphthalein within the pH ranges shown belovw pH O colorless ° pH 83 faintly pink bright fuchsia O neutra O colorless 983pH 10faintly pink 10 - bright fchsia O colorless O bright fuchsia 12 - 13- PH 10 O faintly pink 14- Check Answer 0 Next Ext Previous 89 9 ratings pH83 colou … View the full answer Transcribed image text Label the pH scale below indicating the acidity of a solution in the given regions Indicate the color of phenolphthalein within the pH ranges shown below Previous question Next question The pH scale measures how acidic an object is Objects that are not very acidic are called basic The scale has values ranging from zero the most acidic to 14 the most basic As you can see from the pH scale above pure water has a pH value of 7 This value is considered neutral—neither acidic or basic 1 Excess water is added to extracellular fluid compartment 2 Solute concentration of extracellular fluid compartment decreases 3 Water moves into intracellular fluid compartment by osmosis Place in order the steps that occur as dehydration develops 1 Water is lost from extracellular fluid compartment What is pH pH is a measure of hydrogen ion concentration to determine the alkalinity or acidity of a solution If the pH value of a solution is less than 7 it is an acidic solution If the pH value of a solution is greater than 7 it is a basic solution If the pH value of a solution is equal to 7 it is a neutral solution In order to verify this concentration the titration of this acid solution with a solution of a weak base B of concentration C b = 0032 molL -1 is carried out The hydrochloric acid solution previously prepared is added to a volume V b = 20 mL of the basic solution The table below indicates the various values of pH versus the volume V a If pH 7 then the solution is acidic If pH = 7 then the solution is neutral If pH 7 then the solution is basic This is known as the pH scale The range of values from 0 to 14 that describes the acidity or basicity of a solution You can use pH to make a quick determination whether a given aqueous solution is acidic basic or neutral Examine the pH scale in the figure to determine which of the following substances has the highest H+ concentration strawberries A solution is very acidic if it a has a very low pH value b has a high hydronium ion concentration The dissociation of water a releases free H+ ions into the solution Because the pH scale is logarithmic each numerical change represents a 10X change in ion concentration a How many times more acidic is a pH of 3 compared to a pH of 5 b How many times more basic is a pH of 12 compared to a pH of 8 c Explain the difference between a pH of 8 and a pH of 12 in terms of H+concentration If pH 7 then the solution is acidic If pH = 7 then the solution is neutral If pH 7 then the solution is basic This is known as the pH scale The pH scale is the range of value s from 0 to 14 that describes the acidity or basicity of a solution You can use pH to make a quick determination whether a given aqueous solution is Acid Rain and the pH Scale The pH scale measures how acidic an object is Objects that are not very acidic are called basic The scale has values ranging from zero the most acidic to 14 the most basic As you can see from the pH scale above pure water has a pH value of 7 This value is considered neutral—neither acidic or basic The pH scale is something were all familiar with most people will remember it from school chemistry lessons Its the scale used to rank how strong an acid or alkali a solution is The colours associated with each number correspond to the colour that universal indicator turns in solutions of that particular pH If pH 700 then the solution is acidic If pH = 700 then the solution is neutral If pH 700 then the solution is basic This is known as the pH scale The pH scale is the range of value s from 0 to 14 that describes the acidity or basicity of a solution You can use pH to make a quick determination whether a given aqueous pH scale The pH scale pH is a numeric scale which is used to define how acidic or basic an aqueous solution is It commonly ranges between 0 and 14 but can go beyond these values if sufficiently acidicbasic pH is logarithmically and inversely related to the concentration of hydrogen ions in a solution The pH to H + formula that represents this relation is The pH scale is often indicated as a vertical bar graph with scaled numbers from 0 to 14 top to bottom The lower numbers at the top are the more acidic pH while the higher numbers near the 1 pH is a measure of acidity or basicity of an aqueous solution 2 It is a mathematical notation that describes the power of an acid or a base The concentration of the acid is represented as H + or H 3 O + pH = - log H + or pH = - log H 3 O + 3 The pH scale is the range of pH values from 0 to 14 The pH scale measures how acidic or basic a substance is The pH scale ranges from 0 to 14 A pH of 7 is neutral A pH less than 7 is acidic A pH greater than 7 is basic The pH scale is logarithmic and as a result each whole pH value below 7 is ten times more acidic than the next higher value pH Scale - PhET Interactive Simulations The pH Scale ν pH is a measure of the hydronium ion content of a solution ν pH is defined as pH = -logH 3O+ log is log base 10 not ln natural log H 3O+ is given in molar units M ν pH of pure water H 3O+ = 10 x 10-7 M pH = -log10x10-7 = 70 ν pH of last example H 3O+ = 19 x 10-13 M pH = -log19x10-13 = 127 The pH A pH scale is a tool for measuring acids and bases The scale ranges from 0-14 Litmus paper is an indicator used to tell if a substance is an acid or a base The colour of the paper matches up with the numbers on the pH scale to indicate what kind of substance is being tested For example Vinegar is an acid and measures 24 on the pH scale The pH scale with some common examples The pH scale with examples of common solutions and their pH values DownloadView For commercial use please contact us Students investigate the pH level of household substances by testing a variety of common compounds Substances are tested with pH strips and placed on the continuum of the pH scale range of 1 to 14 After testing a solution the student compares the strip color to the scale provided on the container and gives the solution a rating from 1-14
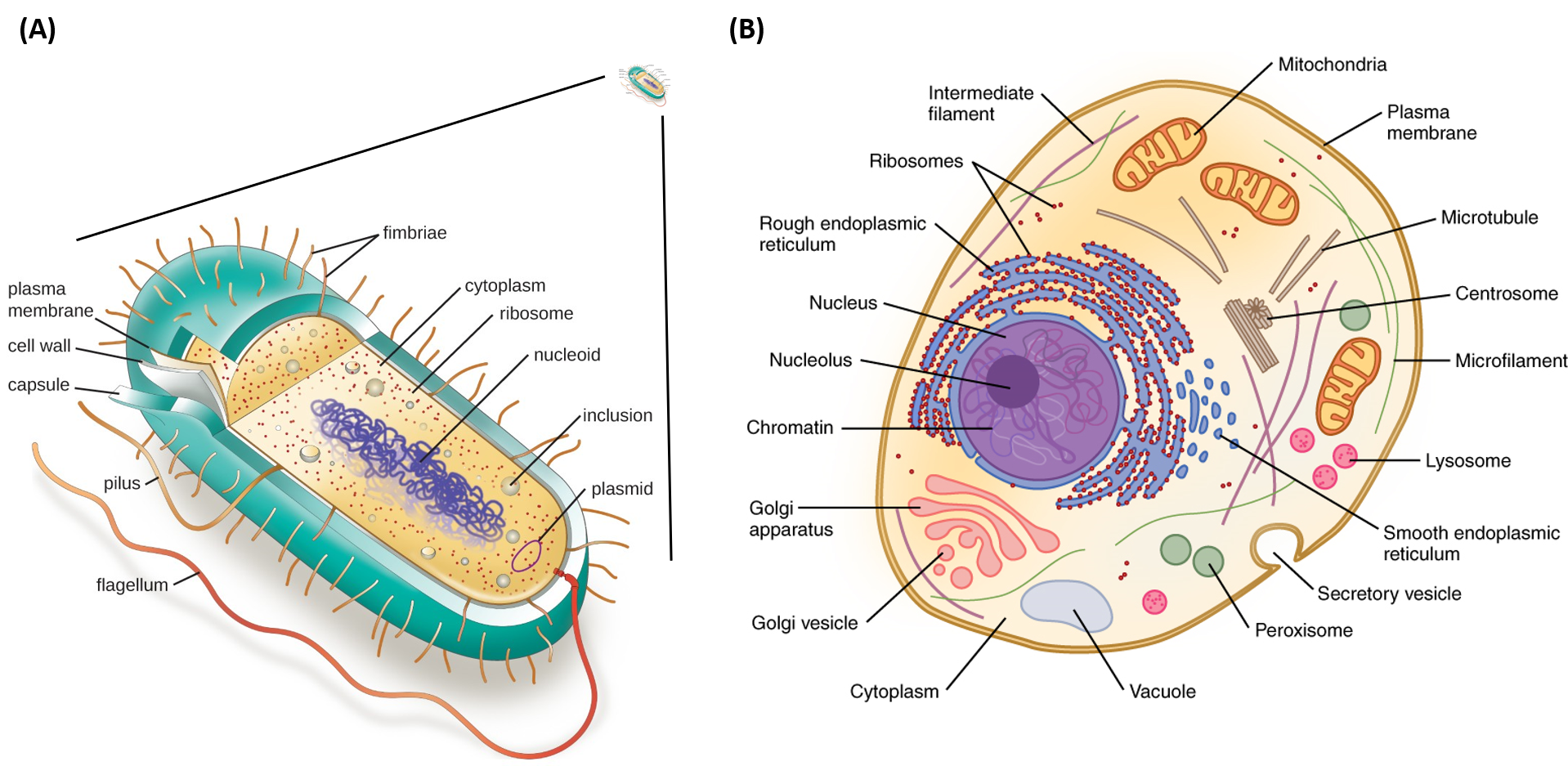
Label the ph scale below, indicating the acidity of a solution in the given regions.
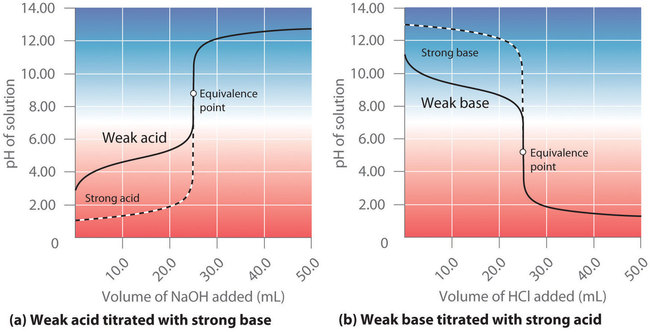














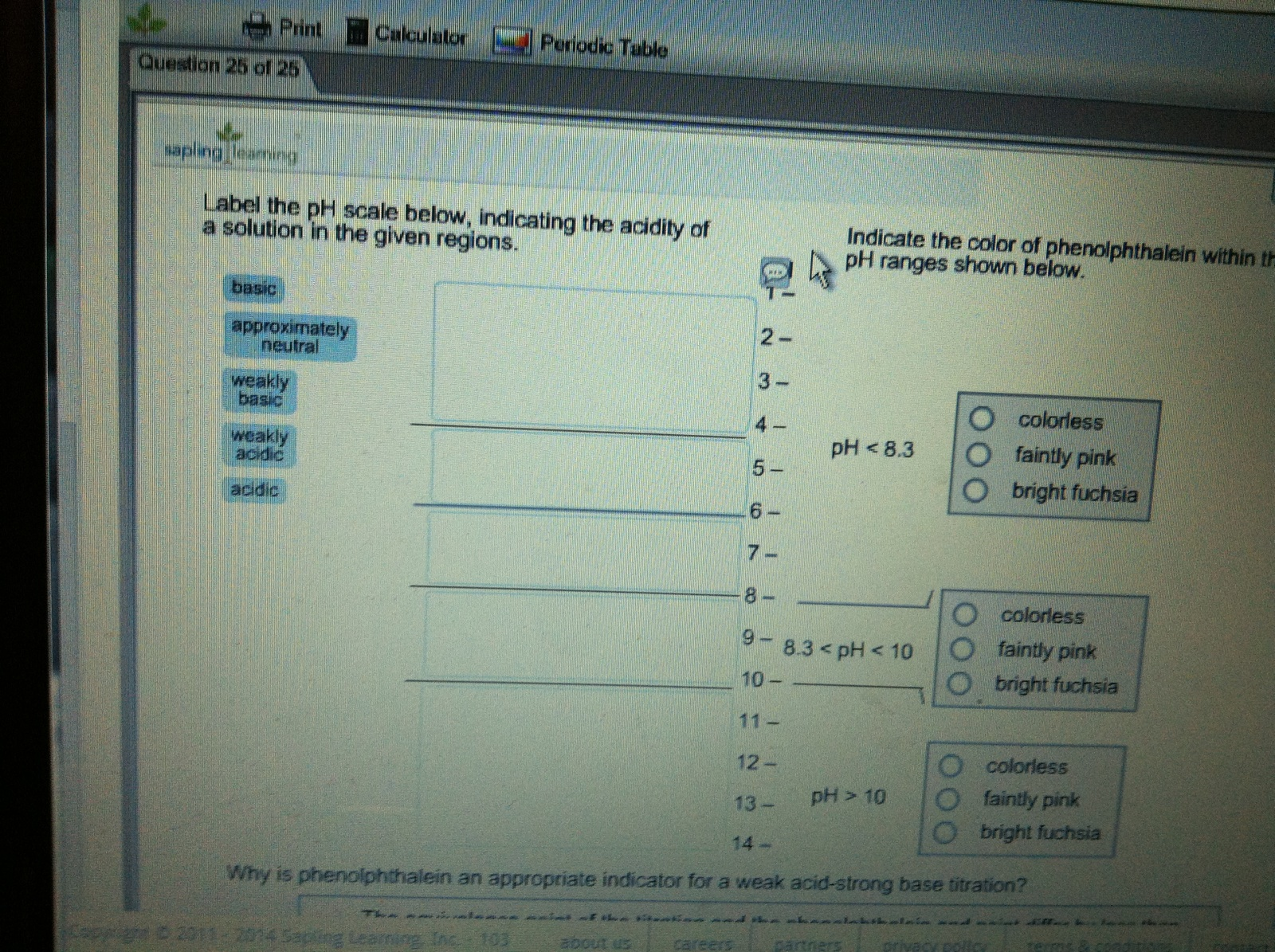

0 Response to "40 Label The Ph Scale Below, Indicating The Acidity Of A Solution In The Given Regions."
Post a Comment