40 What Is Extra Label Drug Usage
Read, understand, and follow entire label of drugs. Know when extra label use is allowed. Explain use, expected outcomes, and risks to clients. Pesticides are mostly OTC products and should be used according to label directions. Problematic Off-Label Drug Use. In most cases, off-label drug use provides more benefit than risk, but it does occasionally cause problems. In the past, large drug manufacturers have been fined by the Justice Department for intentionally marketing their drugs for off-label use, exposing patients and doctors to undue risk.
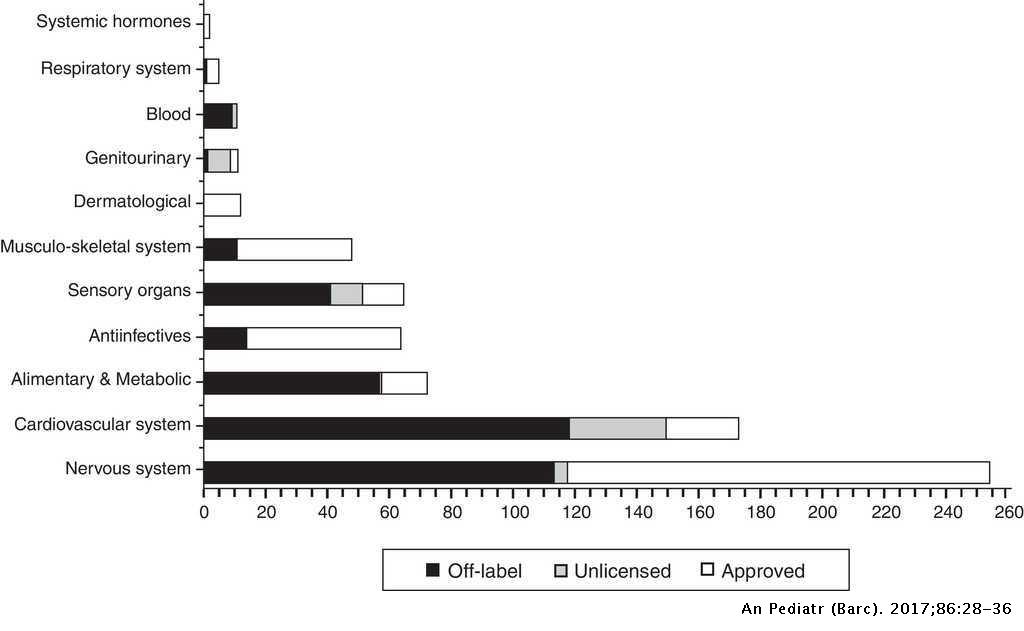
Why Use Drugs Extra-Label at All? There is a need for ELDU, as there are no drugs labelled to treat some conditions in animals. For example, small ruminants (goats in particular) have very few drugs that are currently approved for use. However, there are drugs that are approved for other species that can work in sheep and goats, and must therefore be used in an extra-label manner.

What is extra label drug usage
Extra-label drug use, whether actual or intended, occurs when the drug is used in a manner not in accordance with approved label directions. This includes but is not limited to a different dosage, interval, route, indication, or species. In 1994, Congress passed the Animal Medicinal Drug Use Clarification Act (AMDUCA), which legalized extra. Extra-label use (ELU) is defined as the use of a drug product in a manner that is not consistent with what is indicated on the label, package insert, or product monograph of any drug product approved by Health Canada. Reference is also often made to off-label use, where an unapproved drug is used in a manner that has never been approved by a. Extra-label Drug Use Author: The Minnesota Department of Agriculture Subject: Extra-label drug use is defined as giving a drug in a different way than is described on the FDA-approved label. This is also sometimes called "off-label". Keywords: extra-label drug use, off-label drug use, withdrawal times, VCPR Created Date: 2/8/2021 3:51:15 PM
What is extra label drug usage. Extra-label drug use, whether actual or intended, occurs when the drug is used in a manner not in accordance with approved label directions. This includes but is not limited to a different dosage, interval, route, indication, or species. In 1994, Congress passed the Animal Medicinal Drug Use Clarification Act (AMDUCA), which legalized extra. Extra-label Drug Use Author: The Minnesota Department of Agriculture Subject: Extra-label drug use is defined as giving a drug in a different way than is described on the FDA-approved label. This is also sometimes called "off-label". Keywords: extra-label drug use, off-label drug use, withdrawal times, VCPR Created Date: 2/8/2021 3:51:15 PM An animal drug that is compounded using an approved human or animal drug as the starting material is not adulterated, and using such a drug is considered a legal extra-label use as long as all. Q: Let’s review the definition of extra label drug use. RS: Extra label means using an approved drug in any way that’s not listed on the drug’s label — the species, indications, dosage levels, frequency of treatment, route of administration and anything else on that label. 1. Q: What situations justify use of an extra label drug?
Extra-label use (ELU) is defined as the use of a drug product in a manner that is not consistent with what is indicated on the label, package insert, or product monograph of any drug product approved by Health Canada. Reference is also often made to off-label use, where an unapproved drug is used in a manner that has never been approved by a. But what is extra-label use? It is using a drug: 1) in a food animal species that is not on the label, 2) for an indication that is not on the label, 3) at a dose level, frequency or duration that is not on the label, 4) by a route of administration that is not on the label, 5) in a class of food animal that is not on the label. 9. extra-label drug use of feed additives is not permitted under any circumstances (True/False) extra-label drug use of feed additives is permitted under certain circumstances. FALSE. This is never permitted. What is the standard withdraw time for extra-label drug use? 30 days or more.



























0 Response to "40 What Is Extra Label Drug Usage"
Post a Comment