40 Extra Label Drug Use
But what is extra-label use? It is using a drug: 1) in a food animal species that is not on the label, 2) for an indication that is not on the label, 3) at a dose level, frequency or duration that is not on the label, 4) by a route of administration that is not on the label, 5) in a class of food animal that is not on the label. Aug 18, 2021 · Concomitant Drug Use.. Do not increase your dose or take extra doses of VENTOLIN HFA without first talking to your healthcare provider.... or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such ...
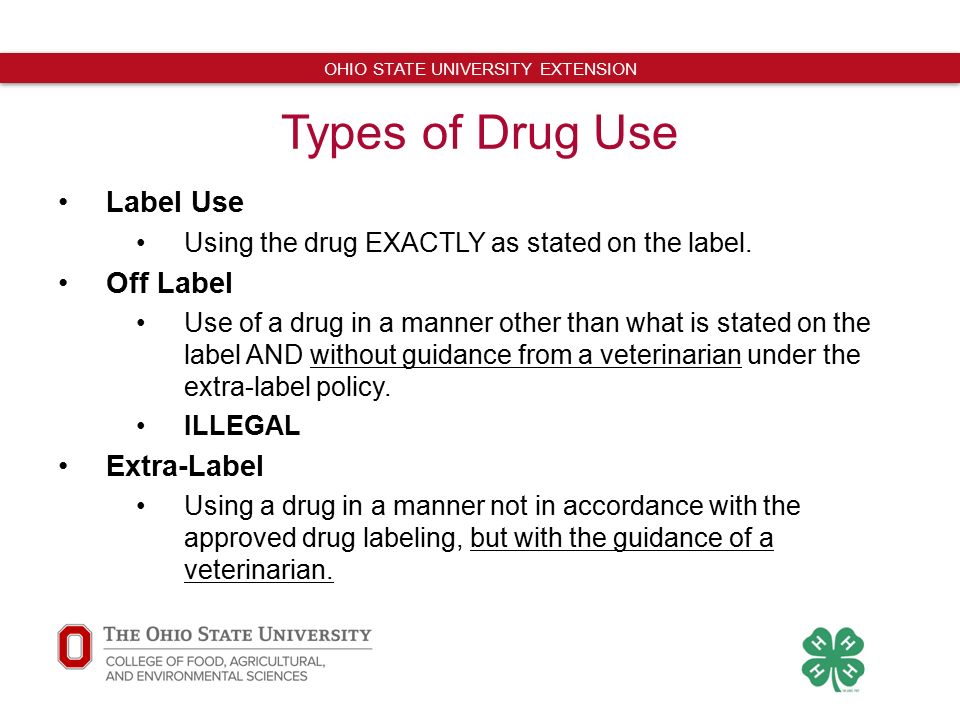
Extra-label drug use refers to the use of an approved drug in a manner that is not in accordance with the approved label directions. ELU of new animal drugs was considered illegal and permitted...

Extra label drug use
A prescription drug (also prescription medication or prescription medicine) is a pharmaceutical drug that legally requires a medical prescription to be dispensed. In contrast, over-the-counter drugs can be obtained without a prescription. The reason for this difference in substance control is the potential scope of misuse, from drug abuse to practicing medicine without a license and without. A drug-eluting stent (DES) is a peripheral or coronary stent (a scaffold) placed into narrowed, diseased peripheral or coronary arteries that slowly releases a drug to block cell proliferation. This prevents fibrosis that, together with clots (), could otherwise block the stented artery, a process called restenosis.The stent is usually placed within the peripheral or coronary artery by an. Extra-Label Drug Use (ELDU) in Animals ELDU, also referred to as "off-label use", refers to the use or intended use of a drug approved by Health Canada in an animal in a manner not in accordance with the label or package insert.
Extra label drug use. Extra-label drug use, whether actual or intended, occurs when the drug is used in a manner not in accordance with approved label directions. This includes but is not limited to a different dosage, interval, route, indication, or species. In 1994, Congress passed the Animal Medicinal Drug Use Clarification Act (AMDUCA), which legalized extra. RS: FDA says extra label drug use is sometimes called off label because the use is "off the label," 10 indicating it considers extra label and off label to be the same. The American Veterinary Medical Association says the term "off label" has no legal or regulatory definition and that extra label is the appropriate term. 11 Extra Label Drug Use is defined by the United States Food and Drug Administration as: "Actual use or intended use of a drug in an animal in a manner that is not in accordance with the approved labeling.This includes, but is not limited to, use in species not listed in the labeling, use for indications (disease and other conditions) not listed in the labeling, use at dosage levels. Extra-Label Drug Use (ELDU) - Position Statement June 30, 2015 Position The CVMA holds that Extra Label Drug Use (ELDU) is an important and legal strategy in the effective and efficient treatment of animals by licensed veterinarians when an approved veterinary product is not available or suitable (1-3).
Extra-label Drug Use Revisited. July 9, 2008 —. The U.S. Food and Drug Administration (FDA) recently approved Baytril® for use in swine but prohibited any extra-label use. Similarly, FDA is proposing to ban the extra-label use of cephalosporins as well adding this class of drugs to the prohibited list. Drugs on the prohibited list cannot be. Q: Let’s review the definition of extra label drug use. RS: Extra label means using an approved drug in any way that’s not listed on the drug’s label — the species, indications, dosage levels, frequency of treatment, route of administration and anything else on that label. 1. Q: What situations justify use of an extra label drug? Antimicrobial Drugs Prohibited for Extra-Label Use in Food Animals (Current as of January 5, 2018. Check for updates at this linked resource.) 21 CFR Part 530.41 Drugs prohibited for extralabel use in animals. The following drugs, families of drugs, and substances are prohibited for extralabel animal and human drug uses in food-producing animals. Extra Label Drug Use (ELDU or “Off-Label Use”) is any use of an FDA-approved drug that differs from instructions on the approved product label. Seven Uses that are ELDU: Any of the following drug uses constitutes ELDU in food-producing animal species:
Jul 26, 2021 · Extra-label use means using an approved human or animal drug in a way that isn’t listed on the drug’s label. It’s sometimes called off-label because the use is “off the label.” Sep 27, 2021 · Extra-label Drug Use. Learn about the law, requirements, and restrictions of extra-label drug use. Veterinary Feed Directive. A VFD Order is a written statement from a licensed veterinarian that authorizes a client to use a VFD drug. Species Specific Website. Drug handling and species specific operation information for veterinarians. Extra-label Drug Use Author: The Minnesota Department of Agriculture Subject: Extra-label drug use is defined as giving a drug in a different way than is described on the FDA-approved label. This is also sometimes called "off-label". Keywords: extra-label drug use, off-label drug use, withdrawal times, VCPR Created Date: 2/8/2021 3:51:15 PM Responsibility of Producers: Engage a veterinarian to develop and oversee a drug management program. Maintain records of treated animals. Maintain identification of treated animals. Have a control system in place to prevent contamination of the human food chain. Maintain storage and accounting of drugs on the premises.
Why Use Drugs Extra-Label at All? There is a need for ELDU, as there are no drugs labelled to treat some conditions in animals. For example, small ruminants (goats in particular) have very few drugs that are currently approved for use. However, there are drugs that are approved for other species that can work in sheep and goats, and must therefore be used in an extra-label manner.
Not drugs for production use. Rules apply to dosage form drugs and drugs administered in water. ELDU in feed is prohibited. ELDU is not permitted if it results in violative food residue, or any residue which may present a risk to public health. FDA prohibition of a specific ELDU precludes such use. Record Requirements
These highlights do not include all the information needed to use IMBRUVICA safely and effectively. See full prescribing information for IMBRUVICA.. potential risk to a fetus and to avoid pregnancy while taking the drug (5.7).... Extra capsules of IMBRUVICA should not be taken to make up for the missed dose.
the use of cocaine or other non-opioid drugs of abuse. There are no data that demonstrate an unequivocally beneficial effect of REVIA on rates of recidivism among detoxified, formerly opioid-dependent individuals who self-administer the drug. The failure of the drug in this setting appears to be due to poor medication compliance.
The extralabel use of the drug or class of drugs presents a risk to the public health. A prohibition may be a general ban on the extralabel use of the drug or class of drugs or may be limited to a...
RS: FDA says extra label drug use is sometimes called off label because the use is "off the label," 10 indicating it considers extra label and off label to be the same. The American Veterinary Medical Association says the term "off label" has no legal or regulatory definition and that extra label is the appropriate term. 11
Extra-Label Drug Use (ELDU) in Animals ELDU, also referred to as "off-label use", refers to the use or intended use of a drug approved by Health Canada in an animal in a manner not in accordance with the label or package insert.
The drug information is in large typeface, the pharmacy details are small and at the bottom of the label, and there's room for multiple warnings and instructions on the back of the bottles.
Extra-label drug use is defined as giving a drug in a different way than is described on the FDA-approved label. This is also sometimes called "off-label". Why is it important to use a drug as directed on the FDA-approved label? Approved withdrawal times are based on the label directions, any other use may result in a longer withdrawal
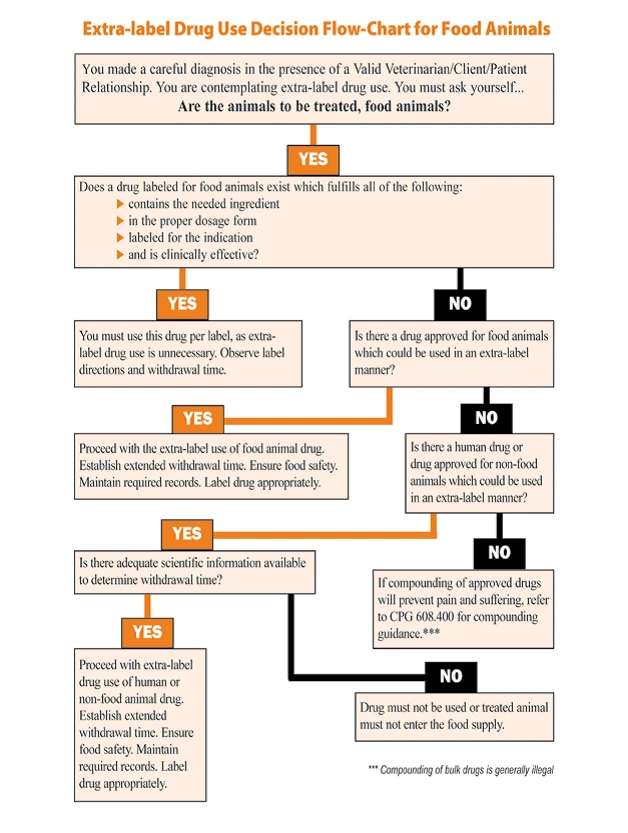
Criteria that must be satisfied to engage in extra-label drug use. 1. Vet/client/patient relationship exists. 2. for therapeutic purposes only. 3. careful diagnosis of condition has been made. 4. It has been determined that there is no other drug specifically labeled that would work for the codition. 5. time period is assigned for drug withdrawal.
§ 530.13 - Extralabel use from compounding of approved new animal and approved human drugs. Subpart C - Specific Provisions Relating to Extralabel Use of Animal and Human Drugs in Food-Producing Animals § 530.20 - Conditions for permitted extralabel animal and human drug use in food-producing animals.
Apr 02, 2020 · Directions for Use Adults and children 12 years and over should take 2 tablets, caplets, gelcaps, or tablespoons every 6 hours as needed with no more than 6 tablets, caplets, or gelcaps in 24 hours. Extra strength Tylenol should not be taken for more than.
What does extra-label mean? The use of a drug in a manner other than that listed on its label Who regulated extra label drug use? Animal Medical Drug Use Clarification Act (AMDUCA) What criteria must be met to use a drug extra-label? 1. vet/client/patient relationship 2. therapeutic purposes only 3. careful diagnosis of the condition
The use of a drug extra label is only for therapeutic purposes or where suffering or death may result from failure to treat This means a drug may not be used in an extra label manner for the purpose of increasing production (i.e. increasing milk production, weight gain of the animal, or for reproduction). Search FDA Approved Animal Drugs
extra-label drug use, which would have virtually. destroyed veterinary medicine. Thus veterinarians. should be careful not to violate the public trust in this.
Extra-label drug use in animals is allowed only in situations where an animal's health is threatened or where the animal may suffer or die without treatment. Before an approved human or animal drug can be prescribed for an extra-label use, one of these general conditions also must be met:
An animal drug that is compounded using an approved human or animal drug as the starting material is not adulterated, and using such a drug is considered a legal extra-label use as long as all.
Extra-label use (ELU) is defined as the use of a drug product in a manner that is not consistent with what is indicated on the label, package insert, or product monograph of any drug product approved by Health Canada.
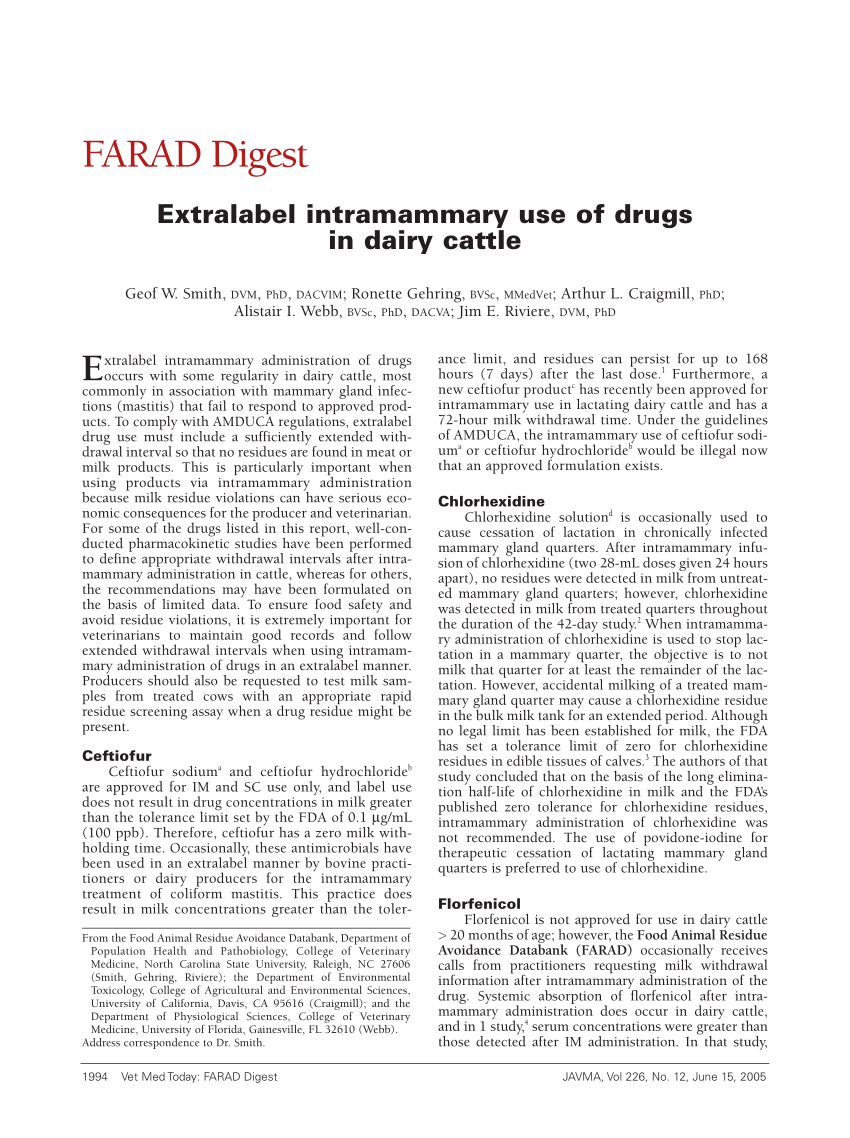
Extra-label drug use in aquaculture. Extra-label drug use in aquaculture. Extra-label drug use in aquaculture J Am Vet Med Assoc. 1993 May 15;202(10):1651-4;discussion 1654-8. Author R Francis-Floyd 1 Affiliation 1 Department of Large Animal.
Extra-Label Drug Use There are few drugs for use in goats that have Food and Drug Administration (FDA) approval. Administering any drug not specifically labeled for use in goats or any product, either prescription or over the counter, that is not used as directed on the label is considered "Extra-label" or "off-label" drug use.
Problematic Off-Label Drug Use. In most cases, off-label drug use provides more benefit than risk, but it does occasionally cause problems. In the past, large drug manufacturers have been fined by the Justice Department for intentionally marketing their drugs for off-label use, exposing patients and doctors to undue risk.
A drug-eluting stent (DES) is a peripheral or coronary stent (a scaffold) placed into narrowed, diseased peripheral or coronary arteries that slowly releases a drug to block cell proliferation. This prevents fibrosis that, together with clots (), could otherwise block the stented artery, a process called restenosis.The stent is usually placed within the peripheral or coronary artery by an.
A prescription drug (also prescription medication or prescription medicine) is a pharmaceutical drug that legally requires a medical prescription to be dispensed. In contrast, over-the-counter drugs can be obtained without a prescription. The reason for this difference in substance control is the potential scope of misuse, from drug abuse to practicing medicine without a license and without.
Interpreting published results of extra-label drug use with special reference to reports of drugs used to correct problem behavior in animals. Hart BL, Cliff KD. J Am Vet Med Assoc, 209(8):1382-1385, 01 Oct 1996 Cited by: 5 articles | PMID: 8870728. Review
Off-label use of a drug or combination of drugs often represents the standard of care. Beta-blockers are another example of beneficial off-label prescribing. Such medications are FDA-approved for.






















0 Response to "40 Extra Label Drug Use"
Post a Comment