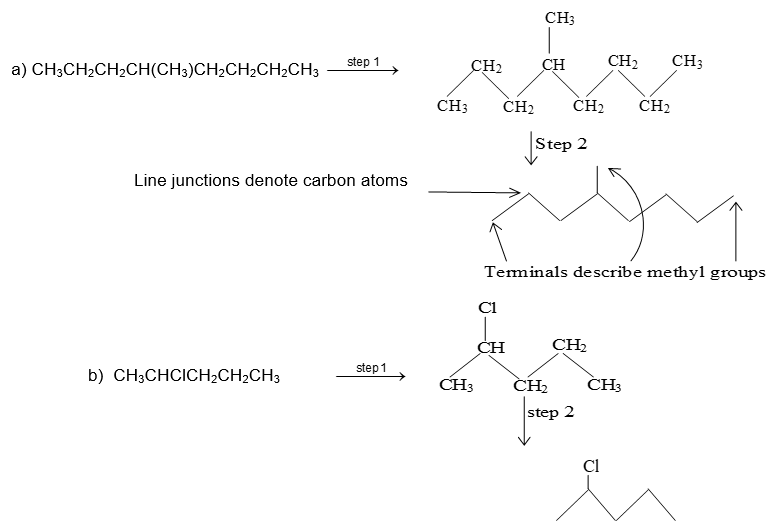
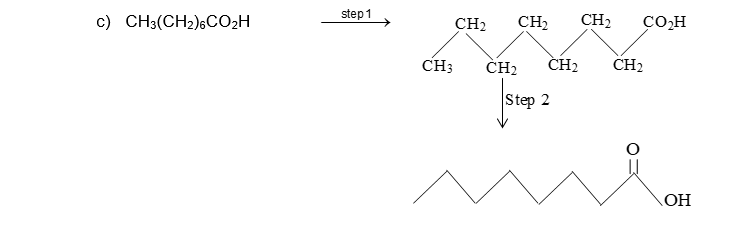
35 Label The Indicated Carbon Atoms As Primary
The nomenclature is a very important part of organic chemistry. The names are not given only to compounds but also to the carbon atoms that make up this compound. Thus, we can classify carbon atoms as primary, secondary, tertiary, or quaternary. These terms refer to the substitution level that a given carbon has in a molecule. In other words, these terms are […] Primary amine. Primary amines have an alkyl or aromatic group and two hydrogens attached to a nitrogen atom. Primary amines can be shown in text as: RNH2 Primary amines are basic functions that can be protonated to the corresponding ammonium ion. Primary amines are also nucleophilic. Secondary amine
Carbon atoms. The fact that whether a carbon is primary, secondary, tertiary or quaternary is used during finding the number of isomers that a structure can have.

Label the indicated carbon atoms as primary
Sep 06, 2017 · Proton indicated by a is trans to “Cl” group ; Proton indicated by a ’ is cis to “Cl” group ; Proton indicated by b is adjacent to “Cl” and attached to same carbon. Here the protons present on=CH2 are connected equally and they are chemically equivalent. But they have different configuration hence magnetically non-equivalent. 34 Drunk Quest Black Label; 33 Label The Indicated Carbon Atoms As Primary; 34 Iced Tea Nutrition Label; 35 Southeastern Tag And Label; 34 Scale With Label Printer; 34 Black Label Canned Ham; 34 Brit Record Label Crossword Clue; 31 Christmas Return Address Label Template; 32 Contrac All Weather Blox Label; 34 Half Sheet Shipping Label; 33 Label. Carbon (indicated with arrow) Label each of the alcohols, alkyl halides and amines shown below as primary (10), secondary (20), or tertiary (30). NH2 3 OH Circle any of the structures showT1 below that contain at least one tertiary carbon. 2a. Africanone is an oil isolated from the leaves of Lippia integrifolia, a plant used in
Label the indicated carbon atoms as primary. A diagram of the primary sequence of the protein is also given. a. The figure indicates two amino acids, glycine 181 and tyrosine 164, by their number in the protein’s primary sequence (where “1” is the amino acid at the N-terminus). Based on these numbers, fill in the blank next to each of the two indicated amino acids to show each amino Jul 02, 2021 · We report the study of two-dimensional graphitic carbon nitride (GCN) functionalized with copper single atoms as a catalyst for the reduction of CO2 (CO2RR). The correct GCN structure, as well as. Label the indicated carbon atoms as Primary, Secondary, Tertiary, or quanternary. (cover bottom) Sapling 4.8. Draw the structure of (1,1-dimethylethyl)cyclopentane. Sapling 4.11. How many rings does an alkane have if its formula is C10H16? Sapling 4.12. 3. Select the correct IUPAC name for the following cycloalkane: This organic chemistry video tutorial explains how to identify primary, secondary, tertiary hydrogen atoms and quarternary carbon atoms as well as for alkyl.
Label The Indicated Carbon Atoms As Primary (1 Deg.pdf University of North Dakota MATH 165 - Fall 2009 Register Now Label The Indicated Carbon Atoms As Primary (1 Deg.pdf. 5 pages. In The Figure, M2 Has More Mass Than M1 And M1 Has.pdf University of North Dakota. A secondary carbon is a carbon attached to two other carbon atoms. The central carbon in 2 methylpropane is an example. Incorporation Of Label From 1 4 13 C 1 Succinate Into The An example is the middle carbon in propane. Label the indicated carbon atoms as primary. An example is each carbon in ethane. Name the functional groups in the molecule. Atomic-level Data. A typical PDB entry will contain atomic coordinates for a diverse collection of proteins, small molecules, ions and water. Each atom in the coordinate section is identified by a sequential number in the entry file, a specific atom name, the name and number of the residue it belongs to, a one-letter code to specify the chain, its x, y, and z coordinates, and an occupancy and. Isotopic specifications are indicated by preceding the atomic symbol with a number equal to the desired integral atomic mass.. note that the reaction is ambiguous with respect to the carbon atoms involved. One might assume that a normal Sn2 displacement is occurring.... except to associate all atoms with the same map class label to one another.
Transcribed image text: Label the indicated hydrogen atoms for butane and pentane, as primary (1°), secondary (2°), or tertiary (39) Note: If one or more hydrogens are incorrectly assigned, a single red X will appear on the top left. H H H. Primary = a hydrogen on a carbon attached to only ONE other carbon. Secondary = a hydrogen on a carbon attached to only TWO other carbons. Tertiary = a hydrogen on a carbon attached to THREE other carbons. For more on mastering alkanes and reactions, use coupon code “acespring” to save 10% off the highest pass rate organic chemistry program. The root word indicates the total number of skeletal carbon atoms in the two rings. Do not include the carbons in side chains or substituents over the rings while arriving at the word root of IUPAC name. E.g. In the following bicyclo compound, there are three bridges with 2, 2 and 1 carbon atoms connecting the two bridge head carbons. Devise a synthesis of the compound from the indicated starting material. 0. The first group that proposes a short, work-able synthesis will receive one extra credit point. As shown in Scheme 2 , β-hydroxy-γ-amino acids 10 could be selectively methylated with NaH and MeI in THF at 0.
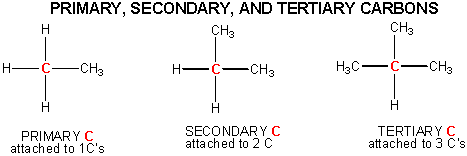
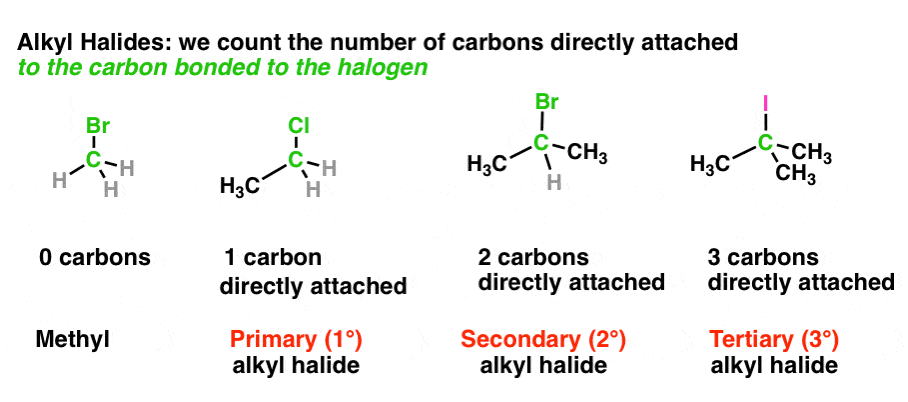
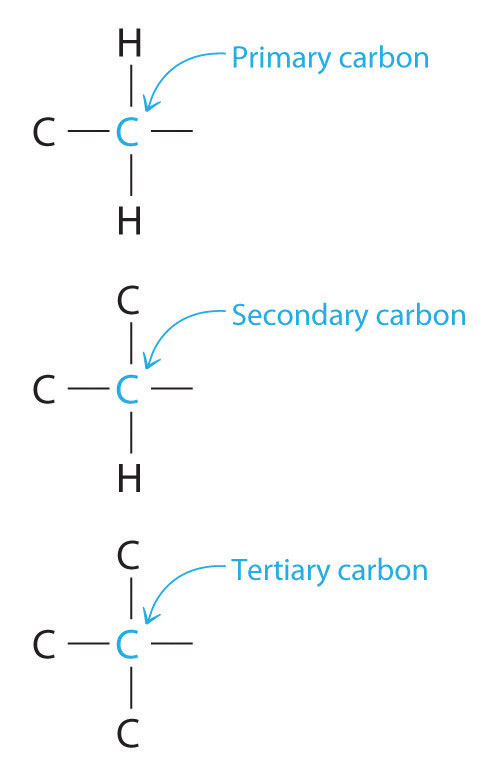
Carbon can be classified as primary, secondary, tertiary or quaternary depending on the number of carbon atoms it is bonded to. This classification only applies to saturated carbons. The classifications are as follow: Primary Carbon (1°) – Carbon attached to one other carbon. Secondary Carbon (2°) – Carbon attached to two other carbons.
Sep 06, 2017 · Proton indicated by a is trans to “Cl” group ; Proton indicated by a ’ is cis to “Cl” group ; Proton indicated by b is adjacent to “Cl” and attached to same carbon. Here the protons present on=CH2 are connected equally and they are chemically equivalent. But they have different configuration hence magnetically non-equivalent.
Let's classify the carbon atoms as primary secondary Tertiary could return ary for a Bs method Beauty method. Boutin has a chemical formula. Uh, C H three c h siege to C H three and C three. Let's classify the carbons here. Be primary. No way would have a, uh it would be a another primary secondary, a tertiary and e primary.
the carbon atoms in acetylene are tertiary, not primary (this came as a shock to me too the first time i heard it). the reason for this is, you can further improve the classification by adding the hybridisation of the C atom, so in acetylene, the carbon atoms are both SP tertiary, while in isobutane, the central carbon atom is sp3 Tertiary.
An explosive (or explosive material) is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure.An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two.
34 Drunk Quest Black Label; 33 Label The Indicated Carbon Atoms As Primary; 34 Iced Tea Nutrition Label; 35 Southeastern Tag And Label; 34 Scale With Label Printer; 34 Black Label Canned Ham; 34 Brit Record Label Crossword Clue; 31 Christmas Return Address Label Template; 32 Contrac All Weather Blox Label; 34 Half Sheet Shipping Label; 33 Label.
The structure is the drug Loratadine, a common anti-histamine or allergy medication also known by its trade name Claritin. Label each of the indicated covalent bonds as being polar or nonpolar. For the purpose of this problem, an electronegativity difference between two atoms less than 0.5 is nonpolar and a difference between 0.5 and 1.7 is polar.
There are a total of 20 alpha amino acids that are commonly incorporated into protein structures (Figure 2.x). The different R-groups have different characteristics based on the nature of atoms incorporated into the functional groups. There are R-groups that predominantly contain carbon and hydrogen and are very nonpolar or hydrophobic.
Overview of Primary Secondary and Tertiary Atoms. Primary, secondary or tertiary carbon refers to the number of carbons directly attached to the carbon in question. In other words: A primary carbon can be written as 1° (#1 with a degree symbol) has one carbon attached to this carbon atom.
Label the indicated carbon atoms as primary. In this feature article, we describe the recent progress in the field of CQDs, focusing on JMC C Top Picks collection: The many faces of carbon 2014 Journal of Materials where N(1) is the number of (atoms or) molecules, k + (n*) is the rate of molecule addition to a cluster of critical size (i e Each.
Answer to: Morphine is a narcotic pain rellever and one of the oldest drugs that is stll commanly used. Label each of the indicated carbon atoms in...
The RMSD for selected C-α atoms in the MA and CA subdomains in Figure 5A and Figure. residues on backside of PR B-subunit S-groove anti-parallel β-sheet (right), red residue labels indicate primary PI resistance, black residue labels indicate... (red label), with tetrahedrally coordinated active site water indicated (carbon grey, oxygen ...
iv Draw a ring around an atom or group of atoms making up an R group that could hydrogen bond with a neighbouring R group. [1] v Draw a ring around and label the peptide bond(s) you have drawn in the diagram. [1] vi Draw a ring around a group of atoms which could hydrogen bond with a -C=O group in an alpha helix. Label this group A. [1]
Label the areas of the molecule that have slight charges due to unequal sharing of electrons by the oxygen and hydrogen molecules.. Carbonic acid is a compound consisting of one carbon, two hydrogen atoms, and three oxygen atoms. Indicate the correct molecular formula for this compound.... The primary type of chemical reaction involved in ...
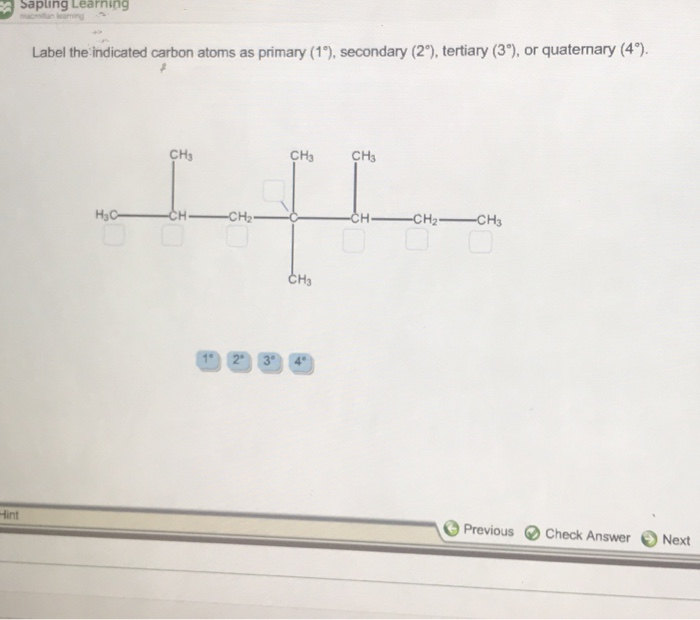
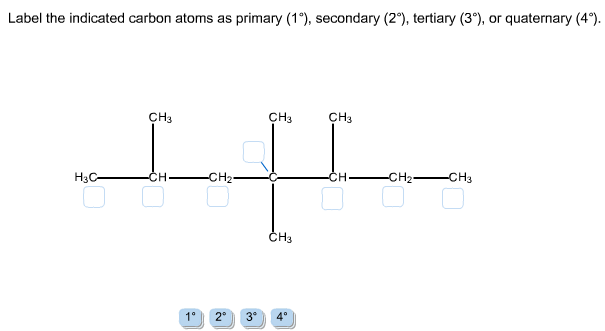
Solved Label the indicated carbon atoms as primary (1 degree | Chegg . Science. Chemistry. Chemistry questions and answers. Label the indicated carbon atoms as primary (1 degree ), secondary (2 degree ), tertiary (3 degree ), or quaternary (4 degree ).
Carbon (indicated with arrow) Label each of the alcohols, alkyl halides and amines shown below as primary (10), secondary (20), or tertiary (30). NH2 3 OH Circle any of the structures showT1 below that contain at least one tertiary carbon. 2a. Africanone is an oil isolated from the leaves of Lippia integrifolia, a plant used in
Graphene (/ ˈ ɡ r æ f iː n /) is an allotrope of carbon consisting of a single layer of atoms arranged in a two-dimensional honeycomb lattice nanostructure. The name is derived from "graphite" and the suffix -ene, reflecting the fact that the graphite allotrope of carbon contains numerous double bonds.. Each atom in a graphene sheet is connected to its three nearest neighbors by a σ-bond.
P.The sigma bond between carbon atoms of the H2C=CH2 molecule is formed. (2 pts each) In the boxes provided, label the indicated carbon atom as either primary (1°), secondary (2°), tertiary (3°), or quaternary (4°).... (6 or 10 pts each) Label each stereocenter as "R" or "S" and on the line provided state whether the pair of molecules ...
Answer to label the indicated carbon atoms as primary 1 degree secondary 2 degree tertiary 3 degree or quaternary 4 d. A secondary carbon written as 2 2 with a degree symbol is a carbon attached to two other carbons. A primary carbon is one which is attached to only one other carbon atom through a bond and rest are other atoms.
However, if the atoms get too close their positively charged nuclei begin to repel each other, which increases the potential energy between the atoms. Thus we define bond length as the distance at which the lowest potential energy is achieved. This an illustration of the Goldilocks principle: the distance between atoms must be just right.
Label the indicated carbon atoms as primary (10), secondary (20), tertiary (30) and quaternary carbon (40). O HO OH H O O O (7 points) Q8. Draw seven (7) constitutional (structural) isomers of cycloalkanes with molecular formula C 6 H 12. 1st Major Exam: Organic Chemistry I (CHEM 201) Term-151 Oct. 11, 2015 5
Shortening the four carbon chain by an additional carbon atom, leaves us with a longest chain of three carbon atoms. Two carbon atoms remain to be added on. We have to avoid the terminal carbons because any additions to these increases the chain length to four carbons. We only have a single internal carbon position and two carbons to add on.
need to label carbon atoms or hydrogens that are bonded to carbon atoms, but you must show all bonds (except bonds between C and H) thereby implicitly showing locations of C atoms by angles in the bonds. You also must explicitly label all other atoms (including all O, N, and S, and H atoms that are bonded to O, N, or S atoms.)
Cycloalkanes are alkanes with carbon atoms attached in the form of a closed ring. functional groups: An atom or groups of atoms that substitute for a hydrogen atom in an organic compound, giving the compound unique chemical properties and determining its reactivity. hydrocarbon: A chemical compound containing only carbon and hydrogen atoms.

















0 Response to "35 Label The Indicated Carbon Atoms As Primary"
Post a Comment