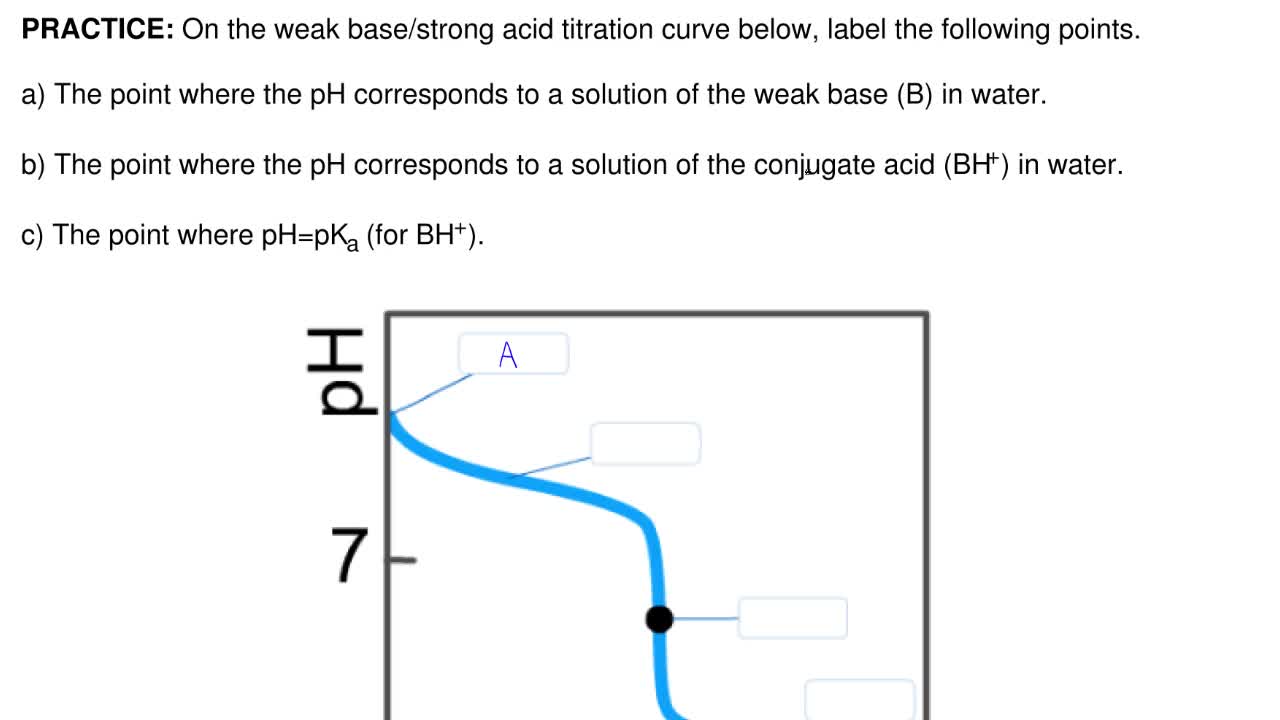
36 On The Weak Base/strong Acid Titration Curve Below, Label The Following Points.
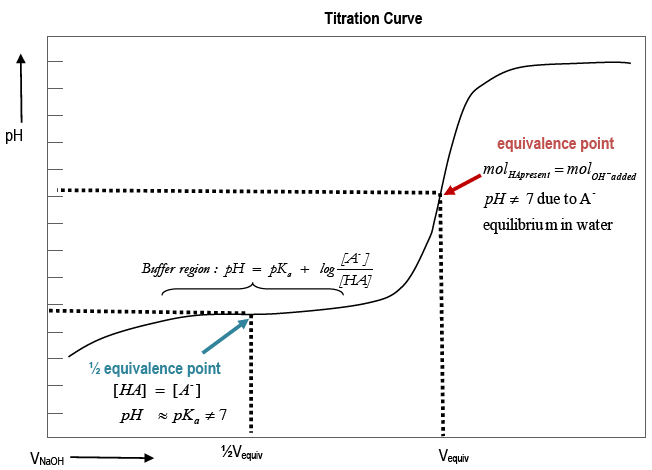
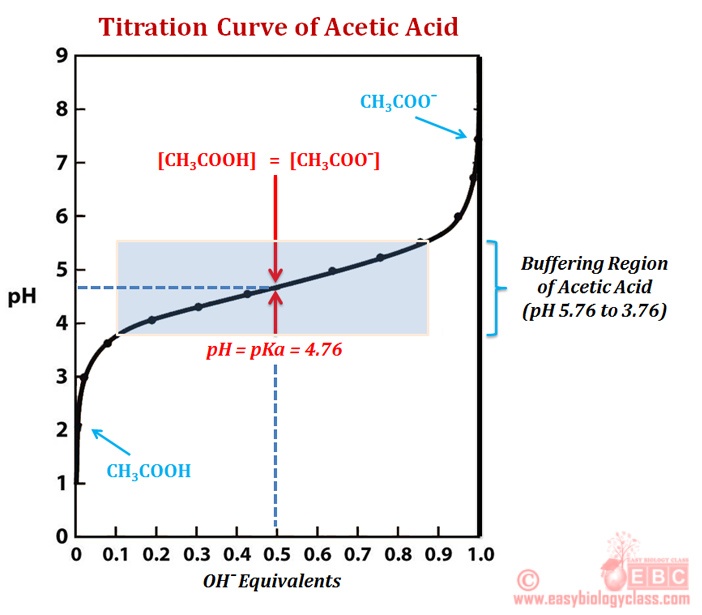
The strong base causes the pH to be greater than 7, & less than 7 for a strong acid/weak base titration What does the half-equivalence point in a weak acid/strong base titration provide us with? pH = pKa because at this point the concentration of the weak acid is equal to the concentration of its conjugate base. reaction of an unknown weak acid, HA, with NaOH: HA (aq) + OH - (aq) → A- (aq) + H 2O (1) An acid-base titration can be monitored either through the use of an acid-base indicator or through the use of a pH meter. Monitoring the pH during titration of a weak acid with a strong base leads to a titration curve, Figure 1.
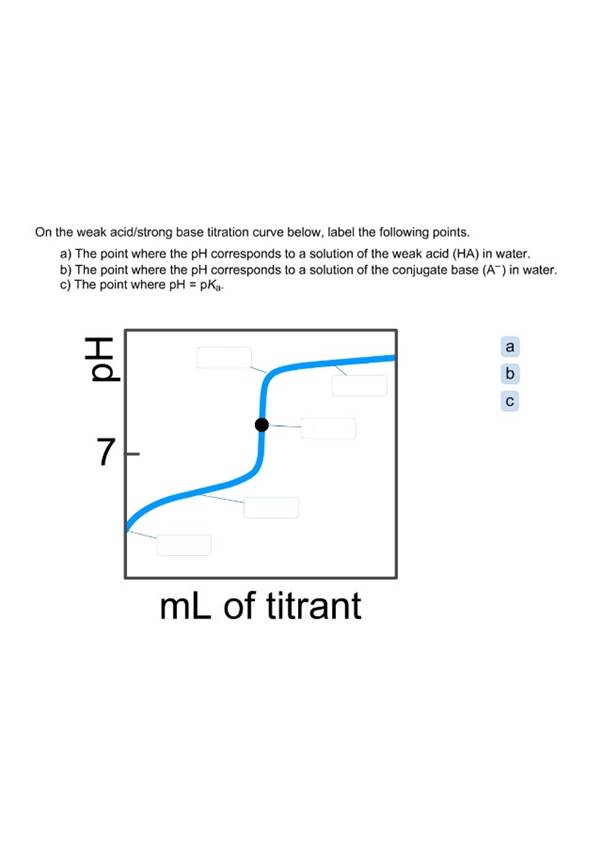
Compare and contrast the titration curves for a strong base-strong acid titration and a weak base-strong acid titration. amethystcockroach626 On the weak acid/strong base titration curve below, label the following points.
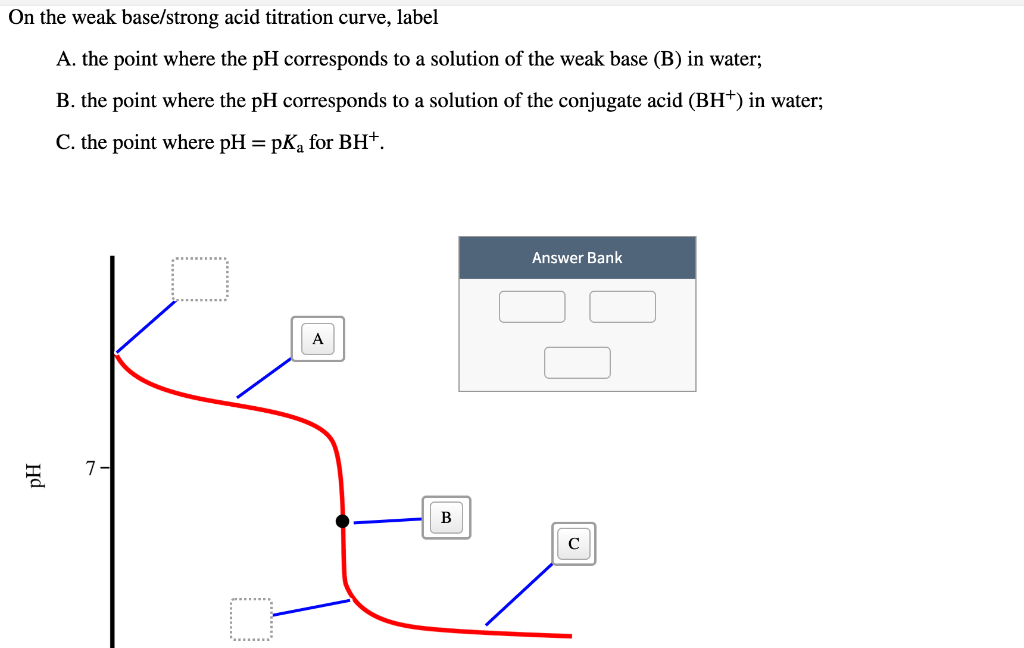
On the weak base/strong acid titration curve below, label the following points.
Transcribed image text: On the weak base/strong acid titration curve below, label the following points. The point where the pH corresponds to a solution of the weak base (B) in water. The point where the pH corresponds to a solution of the conjugate acid (BH+) in water. The point where pH = pKa (for BH+). If the pH of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. All acid titration curves follow the same basic shapes. In the beginning, the solution has a low pH and climbs as the strong base is added. As the solution nears the point where all of the H+ are. On the weak base/strong acid titration curve below, label the following points. a) The point where the pH corresponds to a solution of the weak base (B) in water. b) The point where the pH corresponds to a solution of the conjugate acid (BH +) in water. c) The point where pH=pK a (for BH + ).
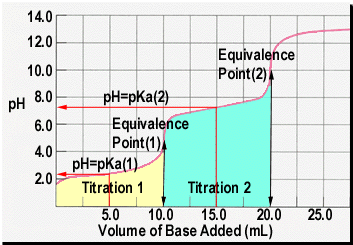
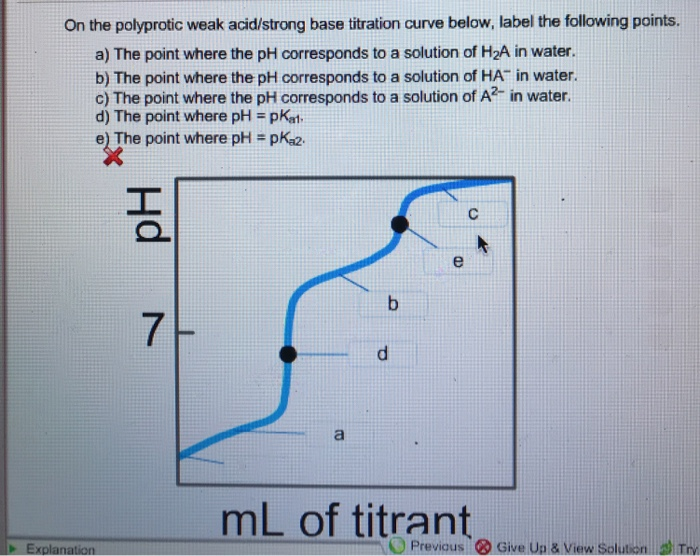
On the weak base/strong acid titration curve below, label the following points.. Transcribed image text: On the weak base/strong acid titration curve below, label the following points. The point where the pH corresponds to a solution of the weak base (B) in water. The point where the pH corresponds to a solution of the conjugate acid (BH+) in water. The point where pH = pKa (for BH+). polyprotic weak acid with strong base titration curve. I have to label the points on a titration curve for a polyproctic weak acid/strong base titration. I have to label the points where; a) The point where the pH corresponds to a solution of H2A in water. b) The point where the pH corresponds to a solution of HA- in water. Key Points. In an acid-base titration, the titration curve reflects the strengths of the corresponding acid and base. If one reagent is a weak acid or base and the other is a strong acid or base, the titration curve is irregular, and the pH shifts less with small additions of titrant near the equivalence point. On the polyprotic weak acid/strong base titration curve below, label the following points. a) The point where the pH corresponds to a solution of H2A in water. b) The point where the pH corresponds to a solution of HA in water c) The point where the pH corresponds to a solution of A n water.
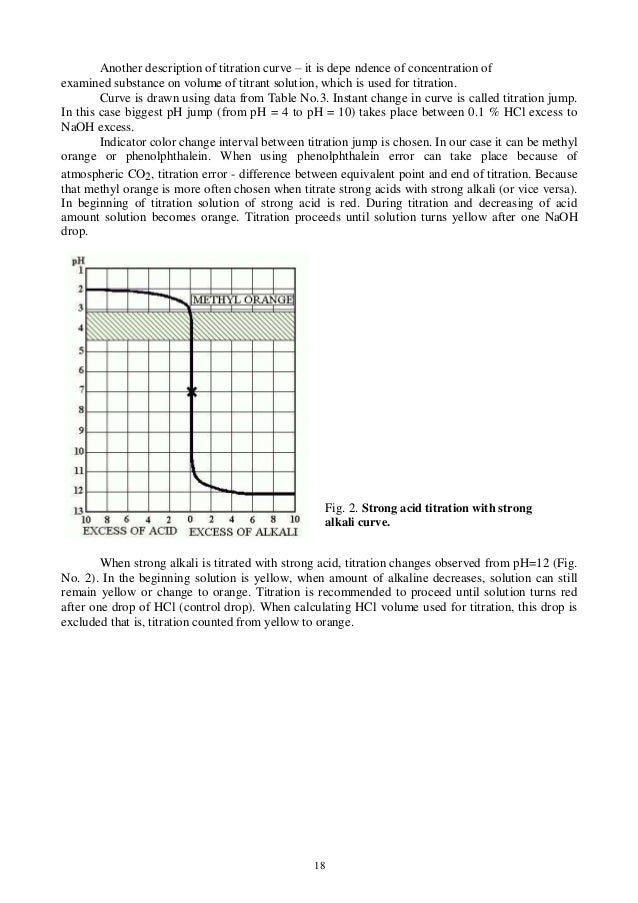
Free essays, homework help, flashcards, research papers, book reports, term papers, history, science, politics 🚀To book a personalized 1-on-1 tutoring session:👉Janine The Tutorhttps://janinethetutor 🚀More proven OneClass Services you might be interested in:👉One... If the pH of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. All acid titration curves follow the same basic shapes. In the beginning, the solution has a low pH and climbs as the strong base is added. As the solution nears the point where all of the H+ are. The simplest acid-base reactions are those of a strong acid with a strong base. Table 4 shows data for the titration of a 25.0-mL sample of 0.100 M hydrochloric acid with 0.100 M sodium hydroxide. The values of the pH measured after successive additions of small amounts of NaOH are listed in the first column of this table, and are graphed in Figure 1, in a form that is called a titration curve.
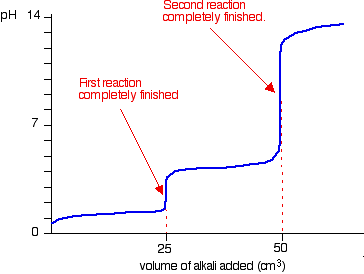
Lab 17 virtual titration lab answers [email protected] In the new exam format for 2007 you should expect a laboratory based situation as A titration is a procedure in which two solutions are introduced to form a reaction that once completed, reaches an identifiable endpoint (Murphy, 2012, p. If you do not have the proper gear, you cannot participate in the lab. Chemistry questions and answers. On the weak base/strong acid titration curve below, label the following points. a) The point where the pH corresponds to a solution of the weak base (B) in water. b) The point where the pH corresponds to a solution of the conjugate acid (BH in water. c) The point where pH- pKa (for BH mL of titrant. Acid base titration lab answers. Acid base titration lab answers using HCl is an example of a diprotic weak base/strong acid titration curve. Because en is diprotic, the titration curve has two equivalence points; the first equivalence point is reached when nM HClH== Cl VM HCle en Vn ne= n where n is the moles of HCl or of en; thus. VV M MV 1 05 05 0 00 0M 25 (0.0 M)(. 0m L).0 mL eq.pt.1 HCl HCl == en = =
On the polyprotic weak acid/strong base titration curve below, label the following points. a) The point where the pH corresponds to a solution of H2A in water. b) The point where the pH corresponds to a solution of HA in water c) The point where the pH corresponds to a solution of A n water. d) The point where pH EpKa1.
Label the important points of the titration curve below.. Titration of a strong acid with a strong base continued. B the point where the ph corresponds to a solution of the conjugate base a in water. Titration curves for weak acid v weak base the common example of this would be ethanoic acid and ammonia. This is the currently selected item.
a for our unknown acid. Analyzing a titration curve-The Importance of the pH at the half-equivalence point. When a strong base (from a buret for example) is added to a weak acid (in a beaker under the buret for example), the strong base will provide hydroxide (OH-) ions. These ions will react with the acid (HA) as in the equation below:
On the polyprotic weak acid/strong base titration curve below, label the following points. a) The point where the pH corresponds to a solution of H 2 A in water. b) The point where the pH corresponds to a solution of HA-in water. c) The point where the pH corresponds to a solution of A 2- in water. d) The point where pH = pK a1.
On the weak base/strong acid titration curve below, label the following points. a) The point where the pH corresponds to a solution of the weak base (B) in water. b) The point where the pH corresponds to a solution of the conjugate acid (BH +) in water. c) The point where pH=pK a (for BH + ).
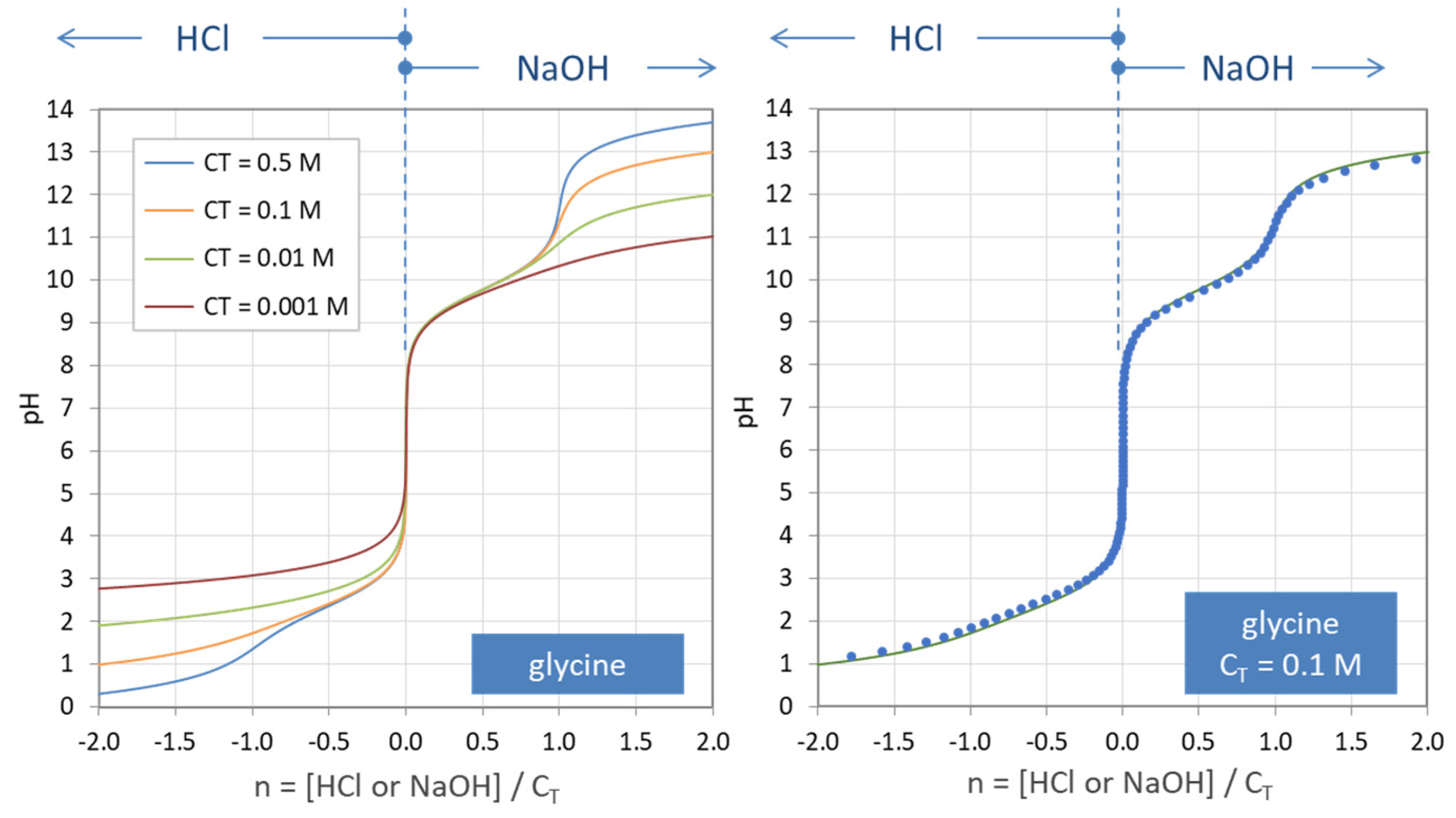
Simple pH curves. All the following titration curves are based on both acid and alkali having a concentration of 1 mol dm-3.In each case, you start with 25 cm 3 of one of the solutions in the flask, and the other one in a burette.. Although you normally run the acid from a burette into the alkali in a flask, you may need to know about the titration curve for adding it the other way around as well.
The following titration curve is characteristic of a weak acid (p Ka = 5.0) and strong base titration. HA ( a q) + OH − ( a q) ⇌ H 2 O ( l) + A − ( a q) Titration Details. 50.00 mL of a 0.1 M weak, monoprotic acid (p Ka = 5) 0.1 M strong base. 25 °C. The initial pH of the solution indicates a weakly acidic solution.
On the weak acid/strong base titration curve, label A. the point where the pH corresponds to a solution of the weak acid (HA) in water; B. the point where the pH corresponds to a solution of the conjugate base (A™) in water; C. the point where pH = pKg. 7- pH Answer Bank В. С A.
Find step-by-step Chemistry solutions and your answer to the following textbook question: Sketch the titration curve for the titration of a generic weak base B with a strong acid. The titration reaction is $$ \mathrm { B } + \mathrm { H } ^ { + } \rightleftharpoons \mathrm { BH } ^ { + } $$ On the curve indicate the points that correspond to the following. the stoichiometric (equivalence) point.
In a weak base-strong acid titration, the acid and base will react to form an acidic solution. A conjugate acid will be produced during the titration, which then reacts with water to form hydronium ions. This results in a solution with a pH lower than 7. An example of this is the titration of hydrochloric acid (strong acid) into ammonia (weak base), which forms the conjugate acid ammonium and.
Associated to on the weak base/strong acid titration curve below, label the following points., Acid reflux disease (gastro-esophageal reflux illness) is an extremely widespread problem that consists of the regurgitation of your belly inside of the esophagus. The disorder generates indicators such as heartburn, throat irritation and agony.
Connected to on the polyprotic weak acid/strong base titration curve below, label the following points., Substantial uric acid results in gout and, possibly, kidney stones. Find out, listed here, what causes superior uric acid, why your diet program is essential, and, the way you can take care of significant acid levels absolutely naturally.
In strong acid-weak base titrations, the pH at the equivalence point is not 7 but below it. This is due to the production of a conjugate acid during the titration; it will react with water to produce hydronium (H 3 O +) ions. In the example of the titration of HCl into ammonia solution, the conjugate acid formed (NH 4 +) reacts as follows:
Equivalence point: point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. At the equivalence point in an acid-base titration, moles of base = moles of acid and the solution only contains salt and water. Diagram of equivalence point. Acid-base titrations are monitored by the change.
The simplest acid-base reactions are those of a strong acid with a strong base. Table 1 shows data for the titration of a 25.0-mL sample of 0.100 M hydrochloric acid with 0.100 M sodium hydroxide. The values of the pH measured after successive additions of small amounts of NaOH are listed in the first column of this table, and are graphed in Figure 1, in a form that is called a titration curve.
(complete titration) half titration The half-way point is important! After you have determined the equivalence point (endpoint) of the titration, go to half that value. The pH at the half-titration point is equal to the pKa of the weak acid, BH+. To get the pKb of the base (B) you MUST subtract the pKa from 14. The
I have to label the points on a titration curve for a polyproctic weak acidstrong base titration. On the weak basestrong acid titration curve below label the following points. A the point where the ph corresponds to a solution of h2a in water. C the point where the ph corresponds to a solution of a2 in water.
On The Polyprotic Weak Acid/Strong Base Titration Curve Below, Label The Following Points. An Arrhenius acid donates a proton ( (H^+)), so a polyprotic acid donates proloads. However, a polyprotic acid differs from a monoprotic acid because it has actually more than one acidic (H^+), so it has the capacity to donate multiple protons.
A titration is a process used to determine the volume of a solution needed to react with a. 14-15 Acids & Bases Review Answers For Later. The titration curve of a strong base/strong acid showed a stretched out curve as it started with a slow gradual change in pH as it reached the equivalence point.
On the weak base/strong acid titration curve below, label the following points.a) The point where the pH corresponds to a solution of the weak base (B) in water.b) The point where the pH corresponds to a solution of the conjugate acid (BH +) in water.c) The point where pH=pKa (for BH+). We are asked to look for the parts described in the.
Answer to saved label the important points on the titration curve below. The point where the ph corresponds to a solution of the conjugate base a in water. 14 12 equivalence point 10 midpoint equivalence point bu. On the weak basestrong acid titration curve below label the following points. For a strong acidbase reaction this occurs at ph 7.














0 Response to "36 On The Weak Base/strong Acid Titration Curve Below, Label The Following Points."
Post a Comment