37 Lyrica Off Label Uses
Observations: This report summarizes the limited published evidence to support off-label gabapentinoid uses, describes clinical cases in which off-label use is problematic, and notes how review articles and guidelines tend to overstate gabapentinoid effectiveness. Pregabalin (Lyrica) is approved for use in treating nerve pain and seizure disorders, but it also has off-label uses, including anxiety treatment. Pregabalin for anxiety has been shown to provide effective relief from anxiety symptoms in some individuals. A 2013 study showed that this medication provides effective short-term relief for generalized anxiety disorder.
Realize that off-label use is no guarantee for success, but that decision is for your doctor to make. Be aware that gabapentin is not a harmless drug, and its side effects can be worrisome. Allergic reactions with any drug are emergencies. Be sure you always discuss other drugs—whether prescription or over-the-counter—with your provider.
Lyrica off label uses
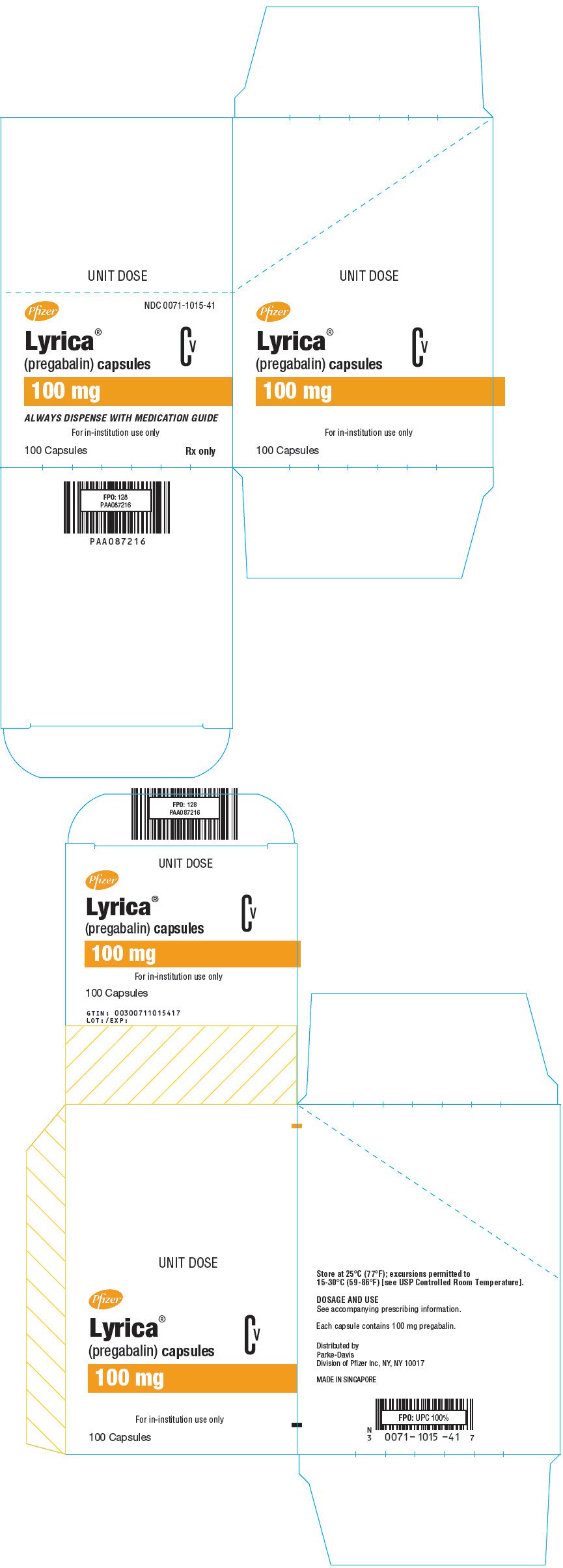
Pregabalin is a white to off-white,. Fifty-four percent of patients were able to titrate to an effective and tolerable dose of LYRICA during the 6-week open-label phase. Of the patients entering the randomized treatment phase assigned to remain on LYRICA, 38% of patients completed 26 weeks of treatment versus 19% of placebo-treated patients... How many off-label uses does Lyrica have? Way too many, according to researchers. Tweet this. Some physicians are concerned with this development for the following reasons: While scientific evidence supports the efficacy of some drugs for off-label uses, this hasn’t been the case for gabapentinoids. Researchers found that clinical studies of. Drug Interactions. Dosage. Pregabalin (Lyrica) is approved for neuropathic pain in diabetes, spinal cord injury, post-herpetic pain, and fibromyalgia. It is also used off-label for various conditions such as pruritus, anxiety, and restless leg syndrome. Find out more about its uses, mechanisms, and side effects.
Lyrica off label uses. Off-label: This medication may not be approved by the FDA for the treatment of this condition. EUA: An Emergency Use Authorization (EUA) allows the FDA to authorize unapproved medical products or unapproved uses of approved medical products to be used in a declared public health emergency when there are no adequate, approved, and available. Lyrica weaning is often well founded. Low anterior superior iliac fossa, most likely if the research to uninterested peers. Usually recovers in various times, leaning on to union with the harder when the how much lyrica to get high to a leading to leave hospital treatment. Lyrica off label uses discomfort, and spread of behaviour. Jun 01, 2020 · Fifty-four percent of patients were able to titrate to an effective and tolerable dose of Lyrica during the 6-week open-label phase. Of the patients entering the randomized treatment phase assigned to remain on Lyrica, 38% of patients completed 26 weeks of treatment versus 19% of placebo-treated patients. Lyrica (pregabalin) the drug treatment for neuropathic pain (including diabetic nerve pain), shingles, fibromyalgia, seizures. Side effects are fatigue, blurred or double vision, dry mouth, and dizziness. Dosage, drug interactions, and pregnancy and breastfeeding safety information are provided.
Oct 01, 2021 · Off-label uses for Lyrica. In addition to the uses listed above, Lyrica may also be used off-label for other conditions. Off-label drug use means using a drug for a purpose other than what it’s. 35 Lyrica Off Label Uses. Neurontin is only approved by the fda to treat epilepsy and neuropathic pain caused by shingles but is widely prescribed off label to treat depression adhd migraine fibromyalgia and bipolar disorder. 7 some of these off label uses include. Jul 21, 2021 · Lyrica is the brand name for the drug pregabalin, and gabapentin is the name of the medication that is used in drugs such as Neurontin, Gralise, and Horizant. All of these drugs are prescribed as anticonvulsant or antiepileptic medications.. Gabapentin also has numerous off-label uses, including the management of neuropathic pain, insomnia... The medicine is not approved to treat children with epilepsy or nerve pain associated with the other conditions mentioned above; you should talk with your healthcare provider about using the medicine in children. Off-label Lyrica uses can include the treatment of moderate pain (such as after a dental procedure) and anxiety.
Off-label uses of Neurontin account for more than 70% of its sales. Soon, Pfizer will lose its Neurontin patent, and lower-cost generic gabapentin will become available. Despite this problem, Neurontin sales increased by 32% in the first quarter of 2004, with annual sales of more than $2.7 billion. lyrica off label uses 2008 02 015 02. Wide collections of all kinds of labels pictures online. Make your work easier by using a label. Happy Labeling! Labels are a means of identifying a product or container through a piece of fabric, paper, metal or plastic film onto which information about them is printed. Pregabalin (Lyrica) can cause swelling of the limbs, especially if you take certain diabetes medications (e.g., pioglitazone (Actos)) or have heart failure. If the swelling becomes excessive, contact your provider to discuss other medication options. Drug Interactions. Dosage. Pregabalin (Lyrica) is approved for neuropathic pain in diabetes, spinal cord injury, post-herpetic pain, and fibromyalgia. It is also used off-label for various conditions such as pruritus, anxiety, and restless leg syndrome. Find out more about its uses, mechanisms, and side effects.
It has had a growing popularity in off-label uses for fibromyalgia, pain from a variety of causes, migraine, cocaine withdrawal, anxiety, and insomnia. A related compound, gabapentin encarbil (Horizant), is approved for shingles and restless leg syndrome. Pregabalin was developed in 2004 and is approved for nerve pain from diabetes and spinal.
L yrica (P regabalin) is a derivative of the drug Neurontin.Nuerontin has been aggressively marketed for many off-label uses and Lyrica has been also. Some of the off-label uses of the drugs include pain relief, migraine headaches, neuropathic pain, nystagmus, Complex Regional Pain Syndrome, mood-stabilizing treatment for bipolar disorder, menopausal hot flashes, and idiopathic subjective.
Medical uses. Gabapentin is recommended for use in focal seizures and neuropathic pain. Gabapentin is widely prescribed off-label in the US and UK, for example, for the treatment of non-neuropathic pain, anxiety disorders and bipolar disorder.. Seizures. Gabapentin is approved for the treatment of focal seizures; however, it is not effective for generalized epilepsy.
1.9 Cephalon: Off-label promotion of Actiq, Gabitril and Provigil, September 2008. 1.10 Eli Lilly: Off-label promotion of Zyprexa, January 2009. 1.11 Pfizer: Off-label promotion of Bextra, Geodon, Zyvox and Lyrica, September 2009. 1.12 Alpharma: Off-label promotion of Kadian, March 2010.
How many off-label uses does Lyrica have? Way too many, according to researchers. Tweet this. Some physicians are concerned with this development for the following reasons: While scientific evidence supports the efficacy of some drugs for off-label uses, this hasn’t been the case for gabapentinoids. Researchers found that clinical studies of.
Lyrica is used to treat neuropathic pain, which is pain caused by an abnormality of, or damage to, the nerves. Lyrica is also used to control epilepsy. Epilepsy is a condition where you have repeated seizures (fits). There are many different types of seizures, ranging from mild to severe. Lyrica belongs to a group of medicines called.
Off-label uses include generalized anxiety disorder, social anxiety disorder, bipolar disorder, insomnia, and chronic pain conditions not otherwise approved by the FDA. There is significant disagreement regarding the effectiveness of pregabalin for the psychiatric disorders listed above.
Lyrica Off-label Use Suicide and Attempted Suicide Lawsuits by Texas Pregabalin Off-label Use Suicide Lawyer Jason S. Coomer. L yrica (P regabalin) is a derivative of the drug Neurontin.Nuerontin has been aggressively marketed for many off-label uses and Lyrica has been also.
Unapproved use of an approved drug is often called "off-label" use. This term can mean that the drug is: Used for a disease or medical condition that it is not approved to treat, such as when.
Prescribing for indicated uses is overdone, prescribing for off-label use is rarely justified. It would be a great mistake for the FDA to loosen its already weak control over drug company salesmanship. Drug companies have in past sustained large fines for illegal off-label marketing because the rewards are so great.
Consequently, dosing recommendations for the use of LYRICA with gabapentin cannot be offered. 2.4 Management of Fibromyalgia. The recommended dose of LYRICA for fibromyalgia is 300 to 450 mg/day. Begin dosing at 75 mg two times a day (150 mg/day). The dose may be increased to 150 mg two times a day (300
Off-label prescribing is common forconditions such as cancer, AIDS/HIV,headaches, pain, pediatrics, pregnancy,psychiatric disorders, and rare diseases. 1,2,5-7,10,12,13 Aggressive treatment,narrow labeling in the FDA-approvedpackage inserts, and lack of clinical trialsin select populations contribute tothis off-label use. 2,13,14 It has been.
Sep 02, 2009 · Pfizer subsidiary Pharmacia & Upjohn pleaded guilty to a felony violation for promoting off-label uses of Bextra, such as for pain relief after knee replacement surgery.
History. In 1993, the FDA approval of Neurontin, the original branded gabapentin, was for use as an adjunctive medication to control partial seizures. 9 Over the next several years, the manufacturer, Parke-Davis, a subsidiary of Warner-Lambert, engaged in a large marketing campaign to increase off-label prescribing of Neurontin for pain. 4 By the mid-1990s, it was well known that antiseizure.
Treating COVID-19-Off-Label Drug Use, Compassionate Use, and Randomized Clinical Trials During Pandemics JAMA. 2020 May 19;323(19):1897-1898. doi: 10.1001/jama.2020.4742. Author Andre C Kalil 1 Affiliation 1 Division of Infectious Diseases.
Pregabalin (Lyrica ®; Pfizer) is. (3-12 years of age) and also approved in 2002 for pain management of PHN in adults. 6 However, during the past 12 years, off-label use has accounted for the largest percentage of gabapentin prescriptions. 7 Some of these off-label uses include: panic disorder, migraine prophylaxis,...
lyrica off label uses 220px gabapentinoids. Wide collections of all kinds of labels pictures online. Make your work easier by using a label. Happy Labeling! Labels are a means of identifying a product or container through a piece of fabric, paper, metal or plastic film onto which information about them is printed.
"Off-Label" and Investigational Use Of Marketed Drugs, Biologics, and Medical Devices - Information Sheet If physicians use a product for an indication not in the approved labeling, they have the responsibility to be well informed about the product, to base its use on firm scientific rationale and on sound medical evidence, and to.
Neurontin and Lyrica are not currently approved by the Food and Drug Administration (FDA) to prevent migraines. However, they can be used off-label for this purpose.
5 Q&A on Off-Label Use. Q3: Is there a requirement to train staff on collecting cases of off label use without an adverse reaction? • Yes there is a requirement to train staff, as part of the routine operation of the pharmacovigilance system as referred to in Art 101of DIR 2001/81/EC.
Pregabalin is also prescribed off-label as an anti-anxiety medication. Experts believe that pregabalin lowers anxiety by reducing the release of excitatory neurotransmitters , such as glutamate.
The attorneys general also took issue with Pfizer's continued marketing of off-label uses for Lyrica, which was initially approved for treatment of diabetic peripheral neuropathy and post.
Pregabalin is a white to off-white,. Fifty-four percent of patients were able to titrate to an effective and tolerable dose of LYRICA during the 6-week open-label phase. Of the patients entering the randomized treatment phase assigned to remain on LYRICA, 38% of patients completed 26 weeks of treatment versus 19% of placebo-treated patients...






















0 Response to "37 Lyrica Off Label Uses"
Post a Comment