35 fda drug label requirements
For prescription drug labeling resources (e.g., Prescribing Information, FDA-approved patient labeling, and carton and container labeling), please see the Prescription Drug Labeling Resources web page
§ 201.24 - Labeling for systemic antibacterial drug products. § 201.25 - Bar code label requirements. § 201.26 - Exceptions or alternatives to labeling requirements for human drug products held by the Strategic National Stockpile. Subpart B - Labeling Requirements for Prescription Drugs and/or Insulin § 201.50 - Statement of identity
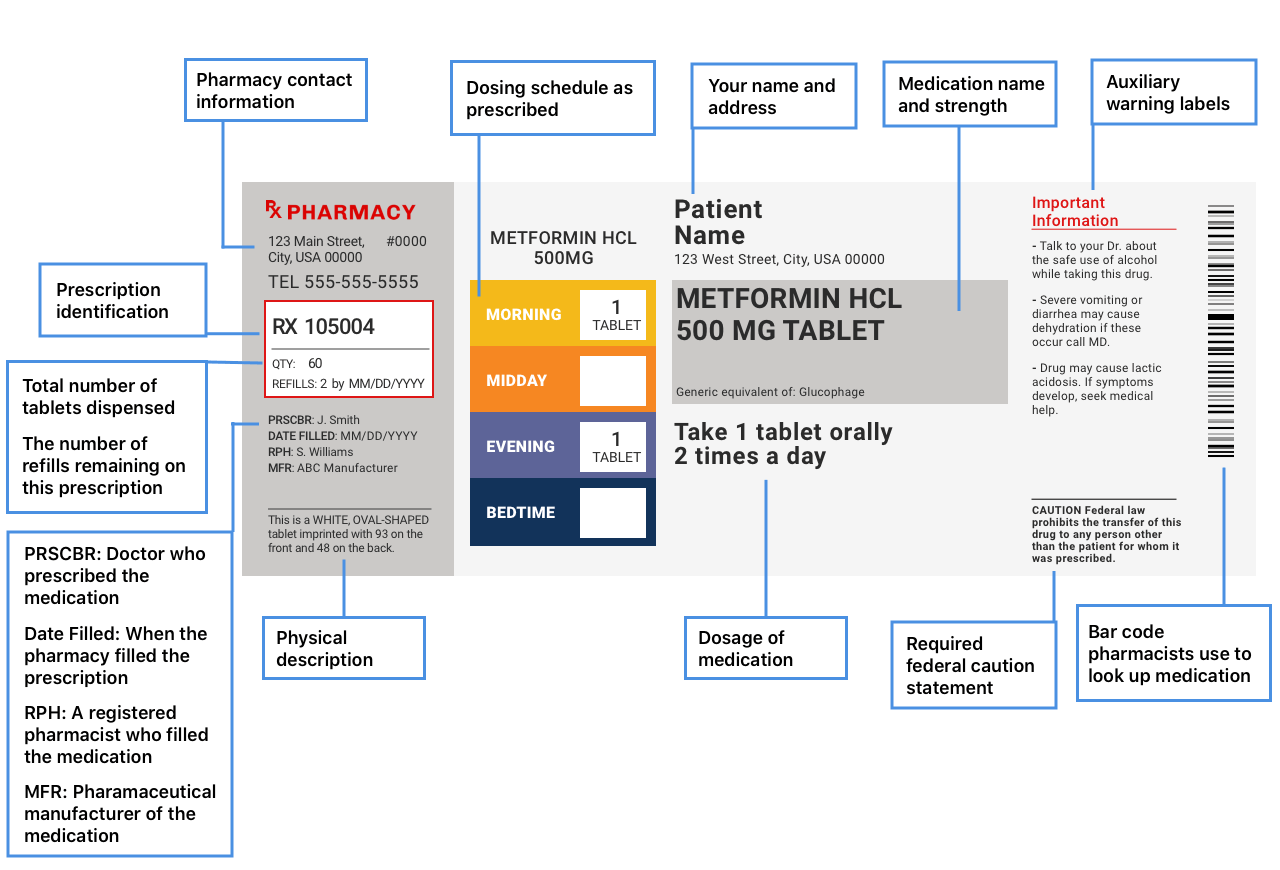
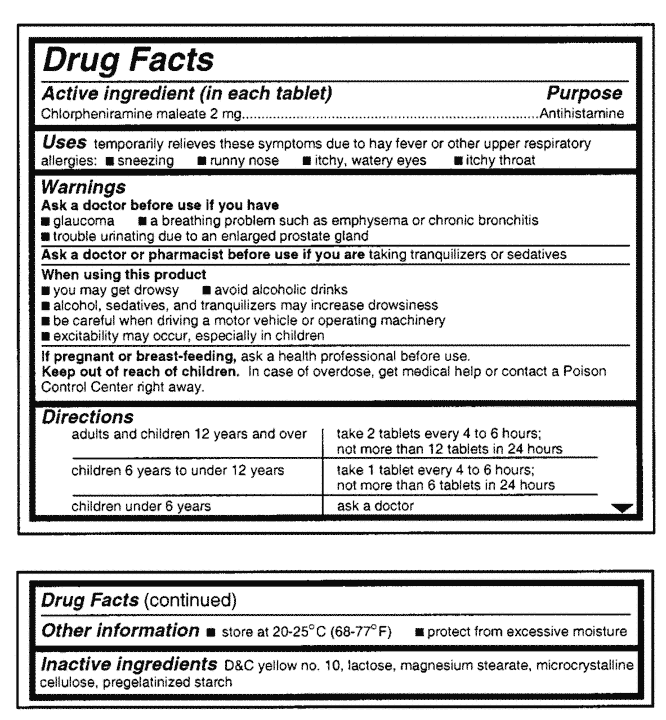
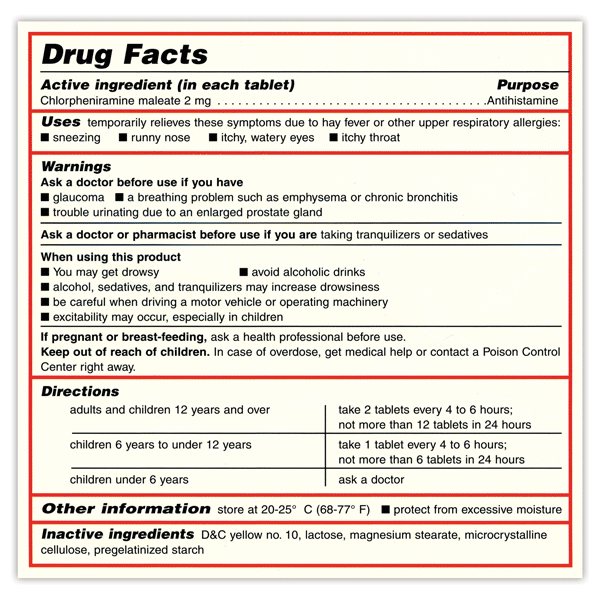
These regulations helped to standardize label format and statement language, making pharmaceutical packaging and product information easier to read and understand. To meet today's FDA regulations, labeling information on drugs must include the following in this order: - Product Name - Drug Facts Table - Active Ingredients - Purpose ...

Fda drug label requirements
FDA Drug listing requirements for API (bulk drugs) Drug establishment registration and drug listing are mandatory requirements for APIs (bulk drugs) commercially marketed in the USA. Manufacturer of API must register their drug establishment where the product is manufactured, packed or processed and list all the drugs which are in the commercial distribution.
Foreign drug establishments that manufacture, repack, re-label or salvage drug products and whose drugs are imported or offered for import into the United States are required to register with the...
To ensure customers have the right information, FDA label requirements for pharmaceutical products are extremely strict and often require specific steps regulated by the FDA.. Since the 1970's War on Drugs movement, which targeted drug reformation, the government has enforced specified requirements for the legal manufacturing and distribution of drug products.
Fda drug label requirements.
FDA Label Requirements & Review FDA Label review is the verification of existing or new labels by our technical experts against FDA's labeling regulation. Product labeling requirements is a confusing and complex process that can include multiple audiences such as patients, physicians and pharmacists.
The Food and Drug Administration (FDA) is amending its regulations governing the content and format of labeling for human prescription drug products (including biological products that are regulated as drugs). The final rule revises current regulations to require that the labeling of new and...
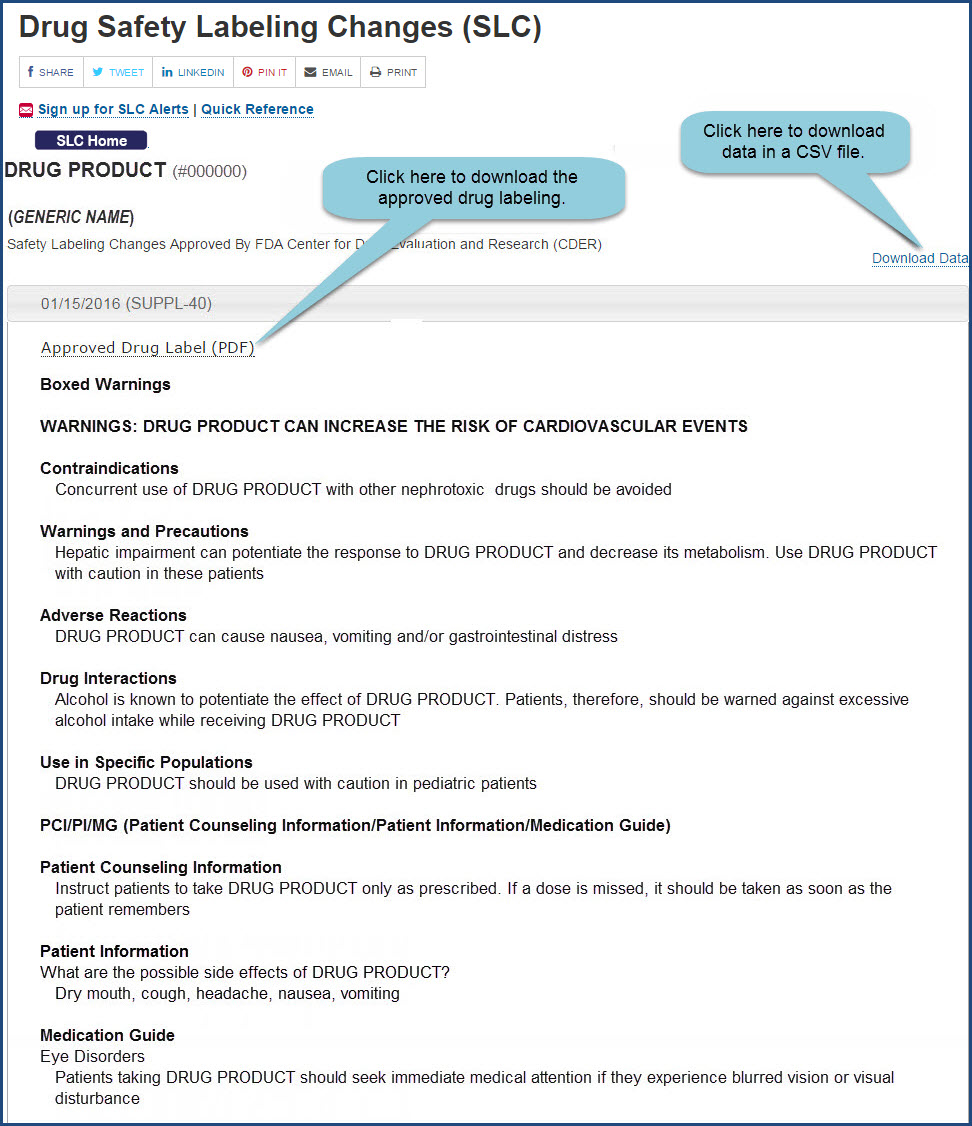
Drug manufacturers and distributors submit documentation about their products to FDA in the Structured Product Labeling (SPL) format. The openFDA drug product labeling API returns data from this dataset. The labeling is a 'living document' that changes over time to reflect increased knowledge about the safety and effectiveness of the drug.
Summary: A new FDA final rule, "Requirements on Content and Format of Labeling for Human Prescription Drug and Biological Products," became effective in June 2006. The rule is part of FDA's ...
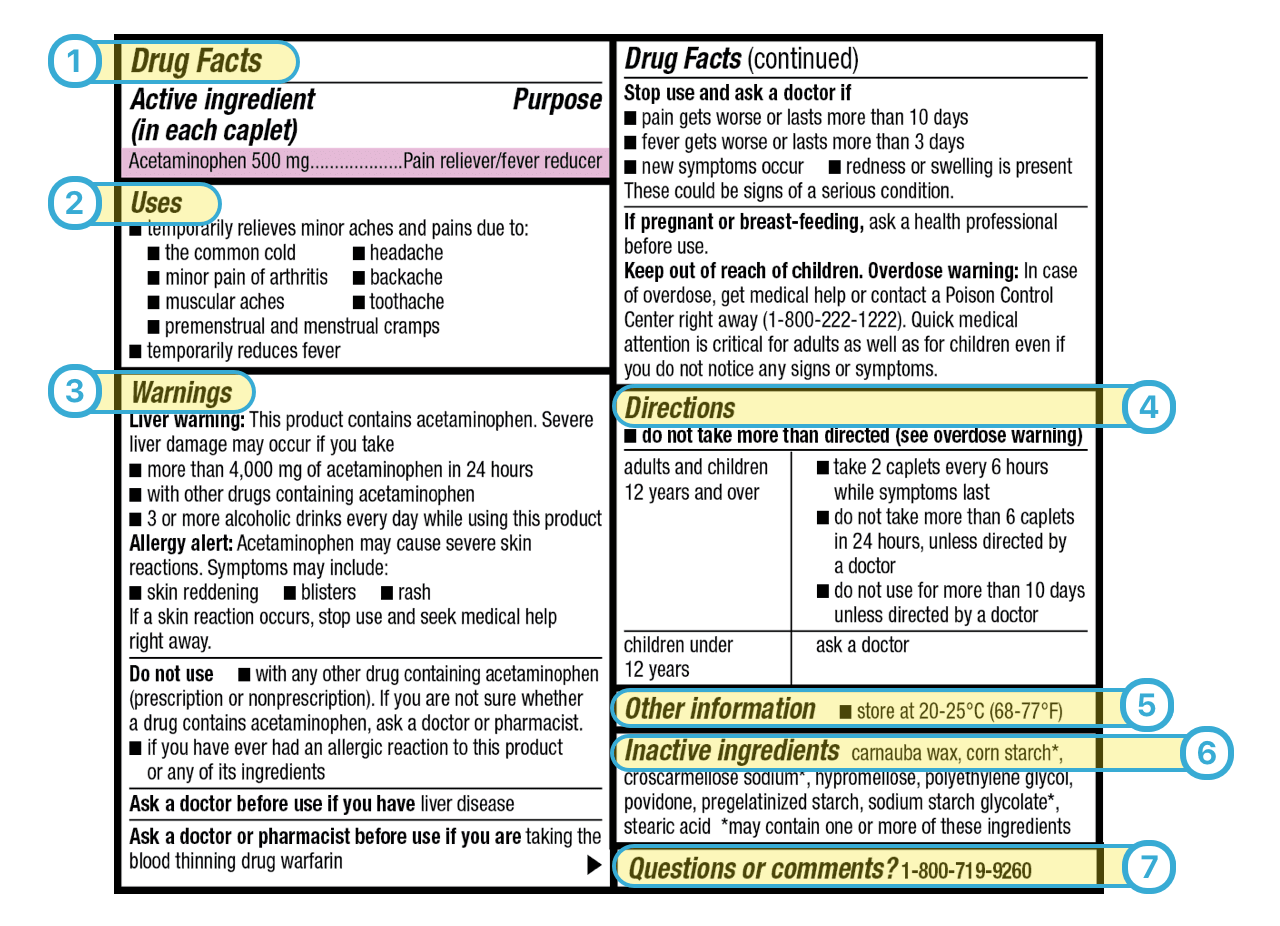
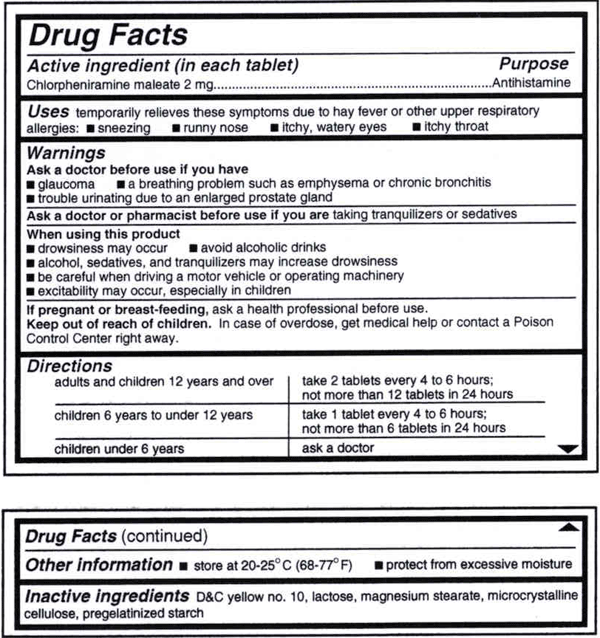
Drug Facts labeling requires, among other things, a description of the active ingredients and their purpose, the product's uses, warnings, directions, other information, and inactive ingredients....
Regulations For Clinical Trial Labeling. An investigational new drug is defined by the Code of Federal Regulations (CFR) as "a new drug or biological drug that is used in a clinical investigation.". In other words, it is any new drug, vaccine, or other biological product for which FDA approval is being sought.
The Food and Drug Administration (FDA) has several FDA drug labeling requirements and regulations that various industries must comply with. In June 2006, a new final FDA regulation, "Prescription Drug and Human Biological Product Content and Format Labeling Requirements," came into effect.
The drug labeling on this Web site may not be the labeling on currently distributed products or identical to the labeling that is approved. Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies described in monographs.
FDA regulations require that certain human drug and biological product labels contain a bar code consisting of, at a minimum, the National Drug Code (NDC) number (21 CFR 201.25).
The U.S. Food and Drug Administration (FDA) requires that all registered medical device and drug facilities renew their FDA registration between October 1 and December 31, 2021. Renew Now. Notice. The U.S. FDA Registration Renewal period CLOSED on December 31, 2020. If you did not renew by the deadline, you must re-register with FDA.
The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented according to the schedule specified in § 201.56(c), except for the requirement in paragraph (c)(18) of this section to reprint any FDA-approved patient labeling at the end of prescription drug labeling or accompany the ...
SUMMARY: "The Food and Drug Administration (FDA) is issuing a final rule establishing a standardized format and standardized content requirements for the labeling of over-the-counter (OTC) drug products. This final rule is intended to assist consumers in reading and understanding OTC drug product labeling so that consumers may use these ...
The new FDA prescription label requirements will include: Clearer, commonly understood terms Abuse-deterrent information Better patient instructions, including visual elements The FDA has a history of tracking abuse and providing new standards or guidelines when widespread abuse is evident.
fda's prescription drug labeling resources website [formerly known as the plr requirements for prescribing information website] provides resources for the development of human prescription drug,...
Appendix A to Part 201 - Examples of Graphic Enhancements Used by FDA: I. Section 201.66 Standard Labeling Format A. Overall 1. The "Drug Facts" labeling is set off in a box or similar enclosure by the use of a barline with all black type printed on a white, color contrasting background.
FDA is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising labeling for already approved prescription drugs. This guidance...
The information on this page is current as of Oct 01, 2021. For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 203.38 Sample lot or control numbers; labeling of sample units. (a) Lot or control number required on drug sample labeling and sample unit label.
Subpart G of these regulations includes the criteria for examining and using materials, issuing labels, using tamper-evident packaging for over-the-counter drugs, conducting packaging and labeling operations, inspecting drug products, and assigning an expiration date.
Labeling, and Dietary Supplements, Center for Food Safety and Applied Nutrition, Food and Drug Administration, 5100 Paint Branch Parkway, College Park, MD 20740-3835, Telephone: (240) 402-2371.
(a) General requirements. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: (1) The labeling must contain a summary of the essential scientific...
FDA Drug Labeling and Ingredient Requirement . Regardless of the manufacturing origin, all of the drugs that are in the U.S. market must comply with the Federal Food Drug and Cosmetic Act (FDCA). 'Intended use' of a drug article is the primary factor in defining which FDA regulations will be applicable for specific drug label.
DEA Schedule (e.g., CII, CIII, CIV, CV) NDC Number (e.g., 1234-5678, 12345-678, 12345-6789) Include the labeler code and product code separated by a dash (first two NDC segments) Package code (third NDC segment) is not required (it will be ignored if included) SET ID: Labeling alphanumeric code (e.g., 0836c6ac-ee37-5640-2fed-a3185a0b16en ...
(e) OTC drug products subject to approved new drug applications. Holders of approved new drug applications for OTC drug products are required under § 314.70 of this chapter to provide the agency with notification of changes in packaging and labeling to comply with the requirements of this section.

















0 Response to "35 fda drug label requirements"
Post a Comment