35 label each reactant and product in the given chemical reaction
in chemical reactions confirm the law of conservation of mass. 11.1 Defining Stoichiometry MAIN Idea The amount of each reactant present at the start of a chemical reaction determines how much product can form. 11.2 Stoichiometric Calculations MAIN Idea The solution to every stoichiometric problem requires a balanced chemical equation.
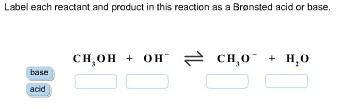
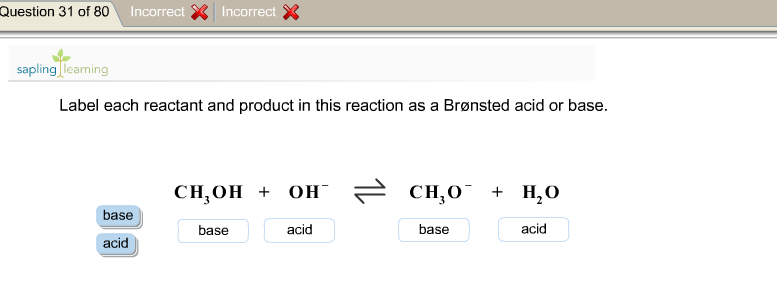
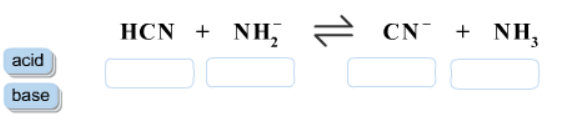
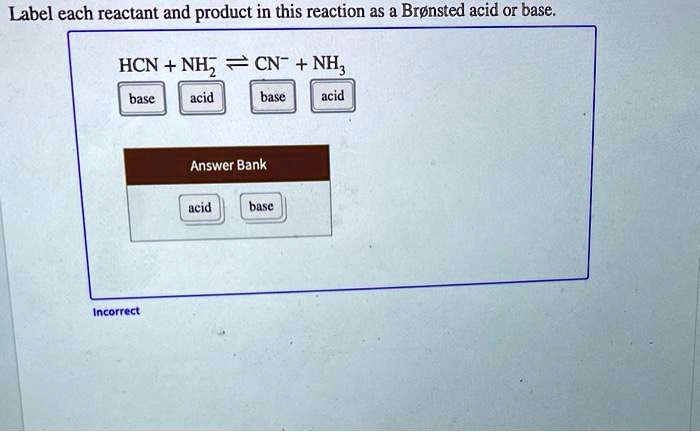
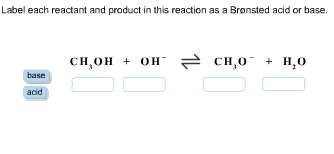
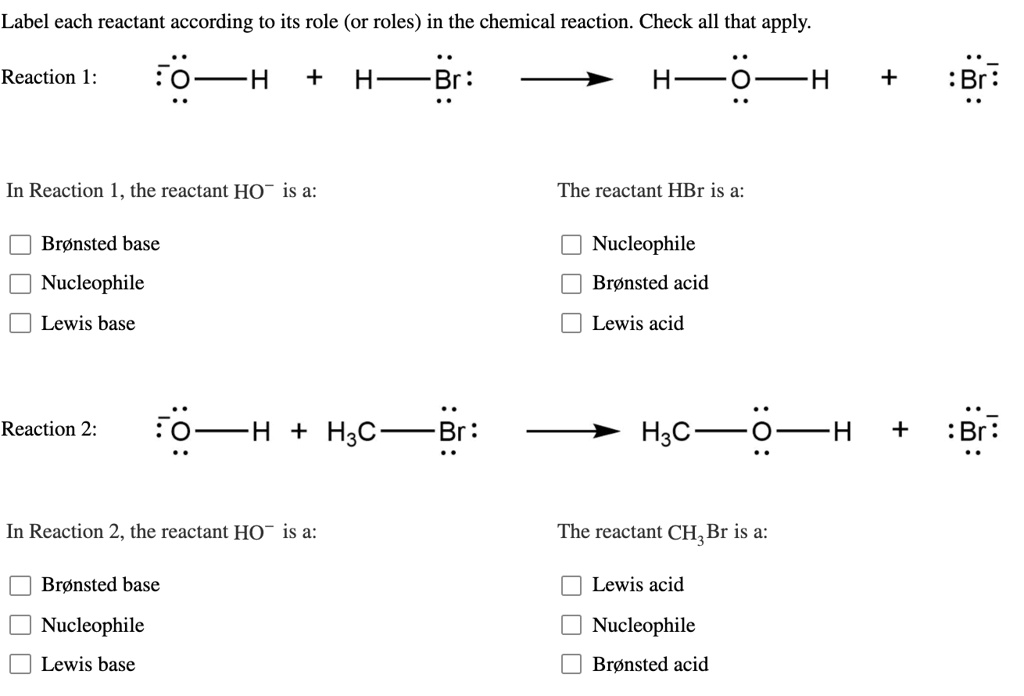
1 Label each reactant and product in this reaction as a Brønsted acid or base. HCN+NH2−↽−−⇀CN−+NH3.
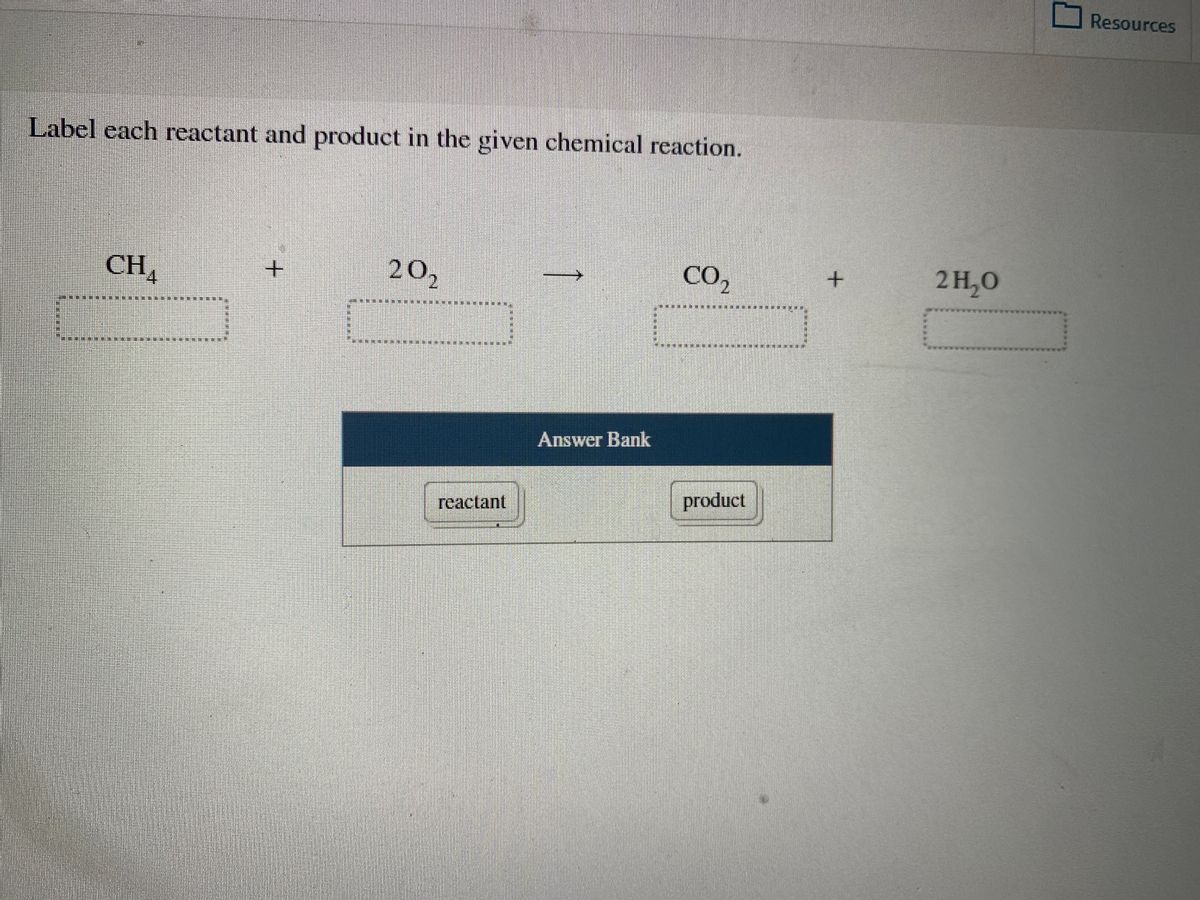
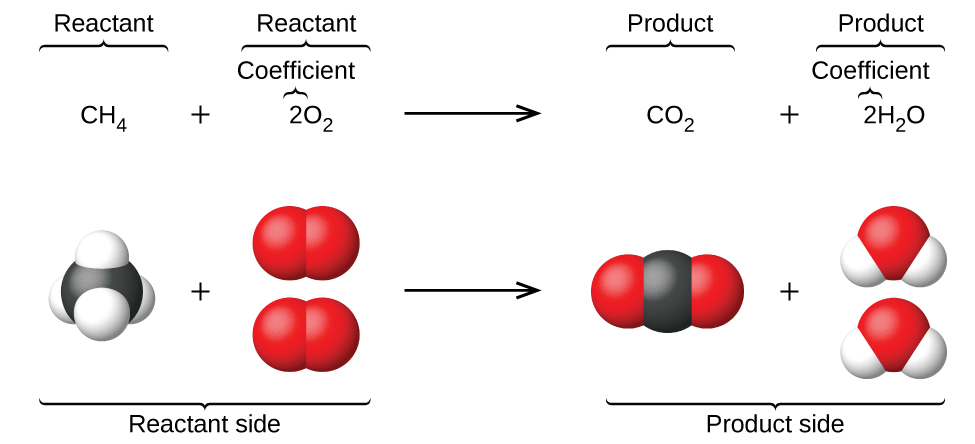
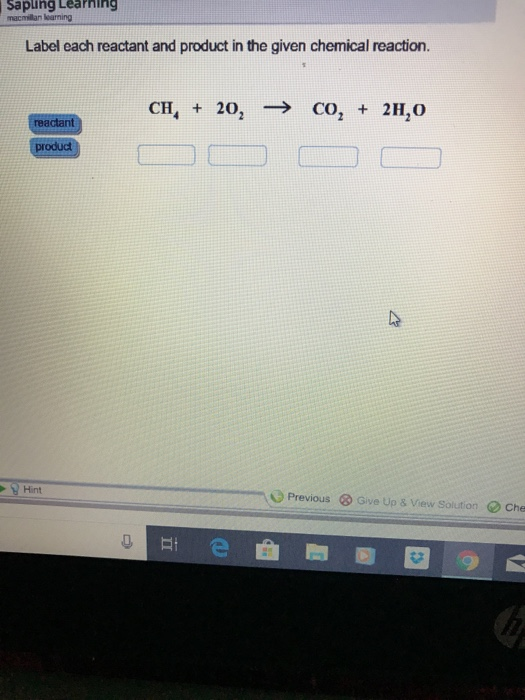
1. Label each reactant and product in the given chemical reaction. CH4 + 2O2 CO2+ 2H2OCH4 + 2O2 CO2 + 2H2O Answer Bank: Product/Reactant 2. Use the law of conservation of mass to answer the questions. Consider a hypothetical reaction in which A and B are reactants and C and D are products.

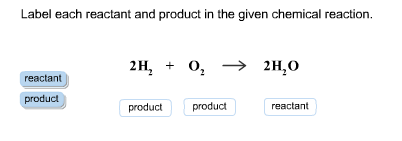
Label each reactant and product in the given chemical reaction
Balance the chemical equation for the chemical reaction. Convert the given information into moles. Use stoichiometry for each individual reactant to find the mass of product produced. The reactant that produces a lesser amount of product is the limiting reagent. The reactant that produces a larger amount of product is the excess reagent.
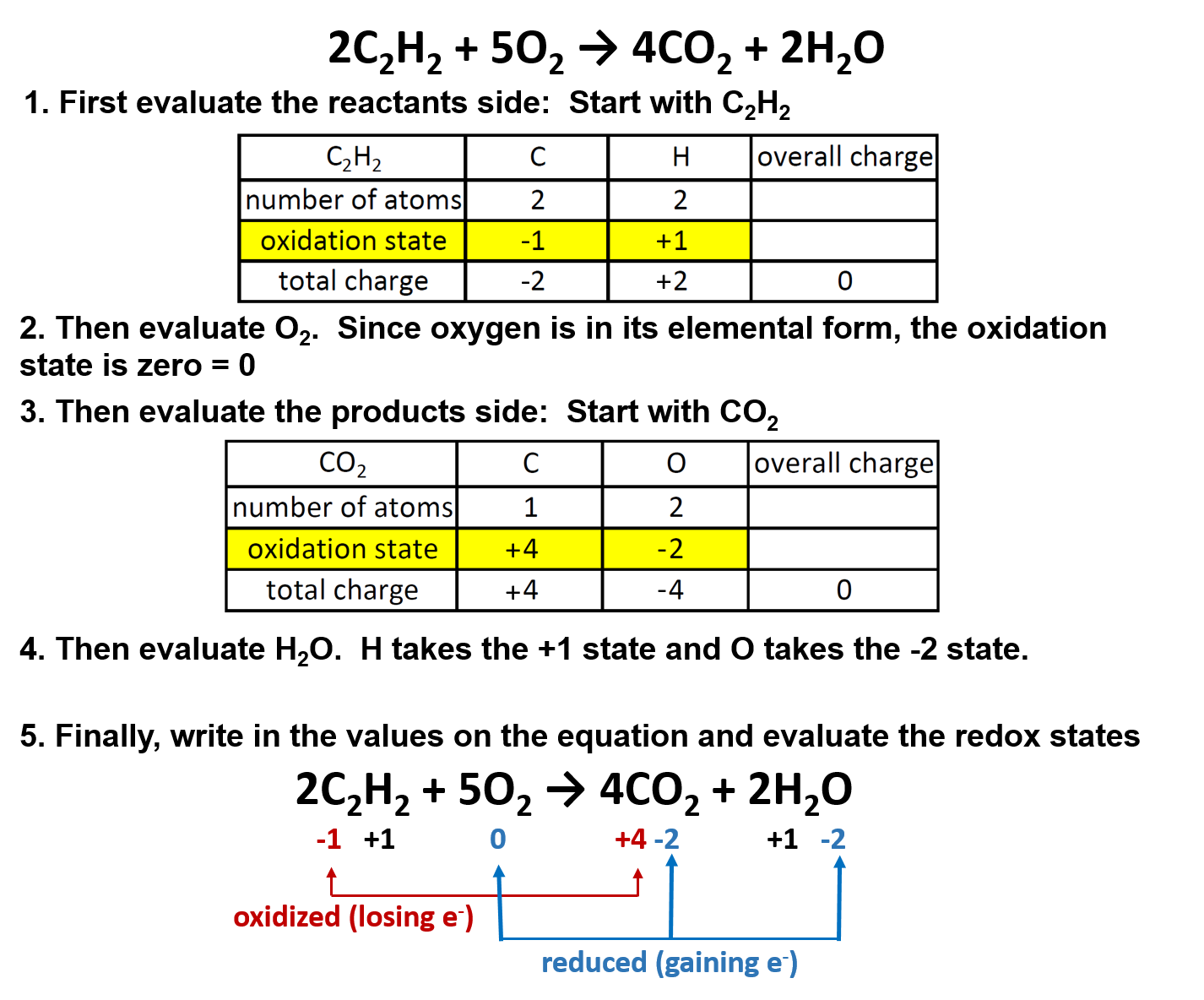
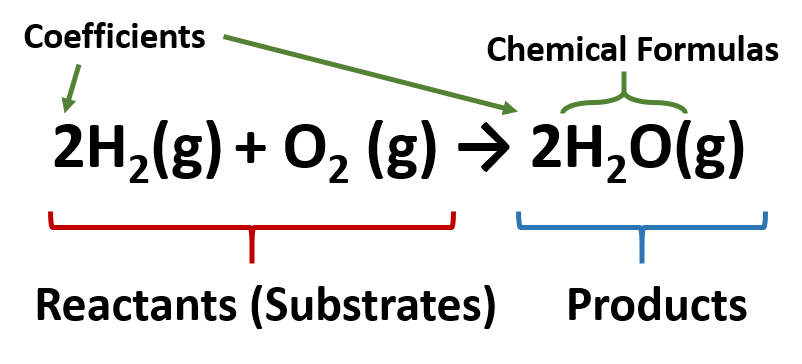
Stoichiometric coefficients are numbers which signify the number of moles of each reactant and product in a chemical reaction. They are placed before the chemical formula of each substance and this can alter the total number of atoms or ions of a particular element on a side of a chemical equation.
Before performing chemical reactions, it is helpful to know how much product will be produced with given quantities of reactants. This is known as the theoretical yield.This is a strategy to use when calculating the theoretical yield of a chemical reaction.
Label each reactant and product in the given chemical reaction.
an expression of a chemical reaction that uses words and symbols to represent the identity of each reactant and product in the chemical reaction The basic types of reactions are: A(n) ________ is a solid product that settles out of an aqueous solution as a result of a reaction between aqueous reactants.
Label each reactant and product in this reaction as a Bronsted acid or base. Write the balanced chemical equation for the reaction of the weak acid HCN with water. Include the phase of each species. Consider three generic acids with the following relative stengths: HX > HY > HZ Rank the strengths of their conjugate bases.
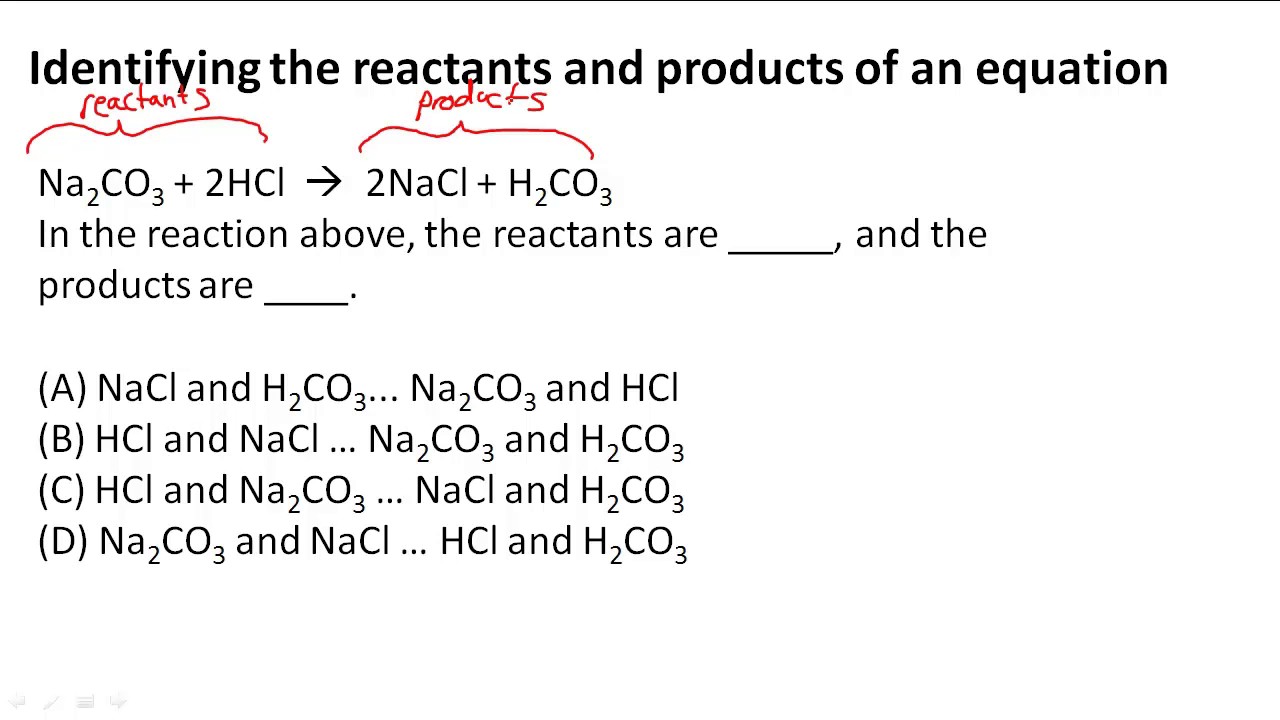
The reactants are on the LEFT HAND SIDE of the equation. The products are on the right hand side. So for the oxidation of iron, #Fe+Srarr FeS# Iron and sulfur are the reactants, and iron sulfide is the product.
2) What is the name given to an expression of a chemical reaction that uses chemical formulas, symbols, and coefficients to represent the identity, physical state, and molar amount of each reactant and product in the chemical reaction?
Label the figure below to investigate the effects of enzymes on chemical reaction rates. 1: Energy needed without enzyme. 2: Energy needed with enzyme. 3: Energy of activation without enzyme. 4: Energy of activation with enzyme. 5: Free energy. 6: Energy of reactant. 7: Energy of product. 8: Progress of reaction.
3. A [physical, chemical] reaction rearranges the atoms that make up the reactant or reactants. After a chemical reaction, [the same, different] atoms are present in the product or products. Atoms [are, are not] destroyed or created, so mass [does, does not] change during a chemical reaction. 4.
The chemical reaction in which two or more products are formed from a single reactant is called ''Decomposition reaction". The reaction in which the place of the ion of a less reactive element in a compound is taken by another more reactive element by formation of its own ions, is called displacement reaction.
The 5 primary types of chemical reactions are: Combination reaction. Decomposition reaction. Displacement reaction. Double Displacement reaction. Precipitation Reaction. 1. Combination Reaction. A reaction in which two or more reactants combine to form a single product is known as a combination reaction.
Mole Ratios. A balanced chemical equation allows us to predict what happens when the reaction takes place. A mole ratio converts moles of one compound in a balanced chemical equation into moles of another compound.. Example: The fireworks that brighten the sky each Fourth of July are based on the reaction between magnesium and oxygen to form magnesium oxide.
Before performing chemical reactions, to get ease you should have to know how much product will be produced with given quantities of reactants. This is said to be as the theoretical yield. This is the phenomenon that use when calculating theoretical yield of a chemical reaction.
Reaction Rate. The rate of a reaction is usually observed by watching the disappearance of a reactant or the appearance of a product within a given time period. Take the chemical reaction: A+2B→ 3C A + 2 B → 3 C. Here, the rate of appearance of product C in time interval Δt is: average rate = ΔC Δt average rate = Δ C Δ t.
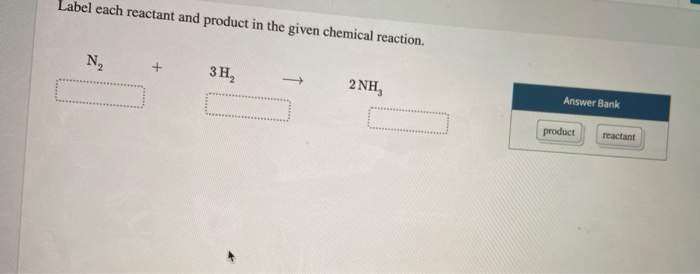
Answer (1 of 3): Reactants are the substances which combine to form different chemical substances. In this case,Hydrogen and nitrogen react together to form ammonia. So,REACTANTS=N2,H2 PRODUCT=NH3 (But if you meant equilibrium condition,then the substance whose concentration reduces with time ...
A chemical bond is a force which holds the atoms together. Therefore, during a chemical reaction, the bonds between atoms have to break so that the atoms can rearrange to form the products. New bonds form between the atoms in the product. Next we will look at a chemical reaction that has been used by humankind for centuries.
The more product that is present at equilibrium, relative to the reactant, the greater the equilibrium constant. (b) The equilibrium constant is given by the concentrations of products over reactants. The following diagrams represent three different systems at equilibrium, all in the same size containers. (a)
Example 1. Write and balance the chemical equation for each given chemical reaction. Hydrogen and chlorine react to make HCl. Ethane, C 2 H 6, reacts with oxygen to make carbon dioxide and water.; Solution. Let us start by simply writing a chemical equation in terms of the formulas of the substances, remembering that both elemental hydrogen and chlorine are diatomic:
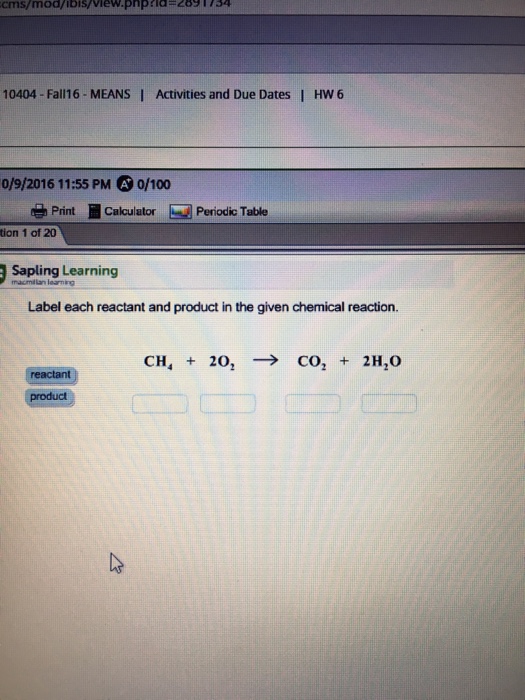
Label each reactant and product in the given chemical reaction. The heat being evolved is the clue that tells you a reaction is taking place. A chemical reaction is a process in which two or more elements or compounds the reactants react together to form one or more new substances the product.
Predict if a reaction will occur when you combine aqueous solutions of iron (II) chloride with aqueous sodium carbonate solution. If the reaction does occur, write a balanced chemical equation showing it. (aq) (aq) PREDICTING REACTION PRODUCTS: DOUBLE REPLACEMENT REACTIONS Using a SOLUBILITY TABLE: Both reactants are soluble SO REACTION OCCURS ...
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. CH4=REACTANT O2 = REACT …. View the full answer. Transcribed image text: Label each reactant and product in the given chemical reaction. CH_4 + 2O_2 rightarrow CO_2 + 2H_2O.
Likewise, the rate of a chemical reaction is a measure of how much reactant is consumed, or how much product is produced, by the reaction in a given amount of time. The rate of reaction is the change in the amount of a reactant or product per unit time. Reaction rates are therefore determined by measuring the time dependence of some property ...
Label each reactant and product in the given chemical reaction. Chemical reaction: A chemical reaction is a process in which two or more elements or compounds (the reactants) react together to form...
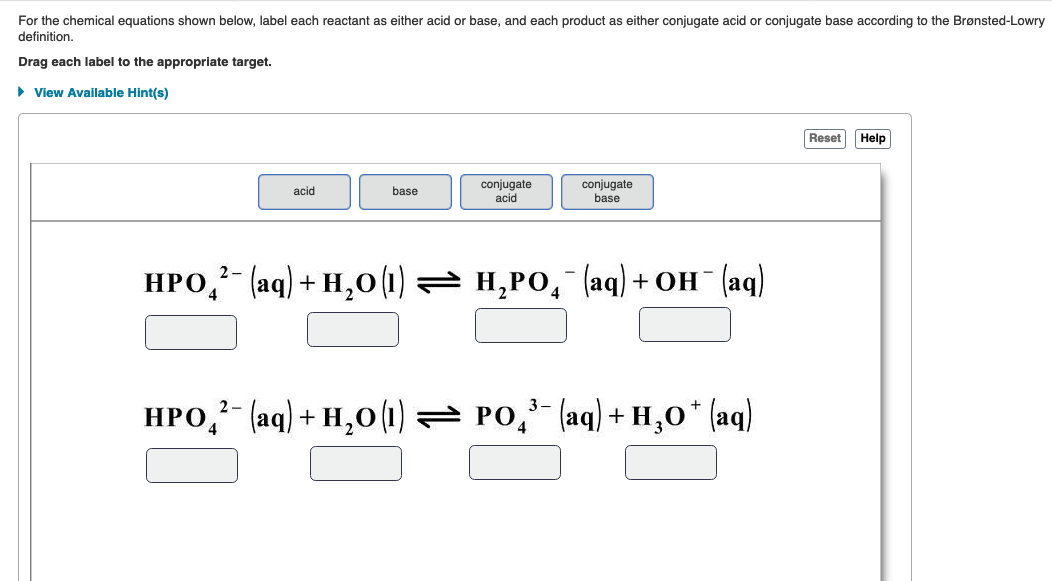
Classification of Chemical Reactions by Type ... and the only requirement is that any given oxidation reaction ... Although both products are written with the (aq) label, the H 2 CO 3 would not be split in writing an ionic equation, becasue carbonic acid is a weak acid, which, as you will learn in more detail later, does not ionize very much ...
Label each reactant according to its role or roles in the chemical reaction. Label each reactant and product in the given chemical reaction. Indeed the very notion of reactant or product has very little to do with chemistry and more with ourselv. In this reaction all reactants and products are invisible.
The representation of a chemical reaction in the form of symbols (substances) is known as chemical equation. A chemical equation consists of reactants, products and an arrow showing the direction of reaction. The equation in which number of atoms of all the molecules is equal on both sides of the equation is known as balanced chemical equation.














0 Response to "35 label each reactant and product in the given chemical reaction"
Post a Comment