36 off label promotion medical device
off-label promotion and materials if companies adhere to FDA's guidance FDA guidelines -- dissemination of off-label information within a CME program is acceptable if the program is independent and non-promotional Key element is independence -- CME content must be free of sponsoring company's influence
Presenting Risk Information in Prescription Drug and Medical Device Promotion (PDF - 933KB) Product Name Placement, Size, and Prominence in Advertising and Promotional Labeling (PDF - 115KB)
are relevant to an individual medical device 3,4), any promotion must be limited to the purposes for which the device has been CE marked. UNDERSTANDING THE PROMOTION OF MEDICAL DEVICES IN THE EUROPEAN UNION Promotion of the off-label use of medical devices is excluded. However, while the promotion of medical devices that have not been CE marked is

Off label promotion medical device
On Promoting Off-Label Use. We've posted more times than we can count in support of the position that FDA-regulated manufacturers should be able to engage in truthful "promotion" of the off-label uses of their products. Well, on nationwide TV - and in the presence of the Commissioner of the FDA - on March 19, 2010 the President of the ...
This medical device training will include a discussion on what constitutes valid scientific evidence to support advertising and promotional claims, a review of relevant Warning Letters to better understand FDA's policies regarding comparisons to competitor products and what constitutes off-label use.
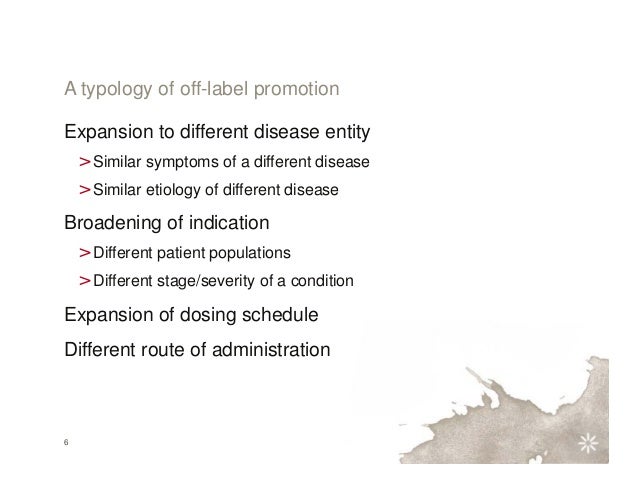
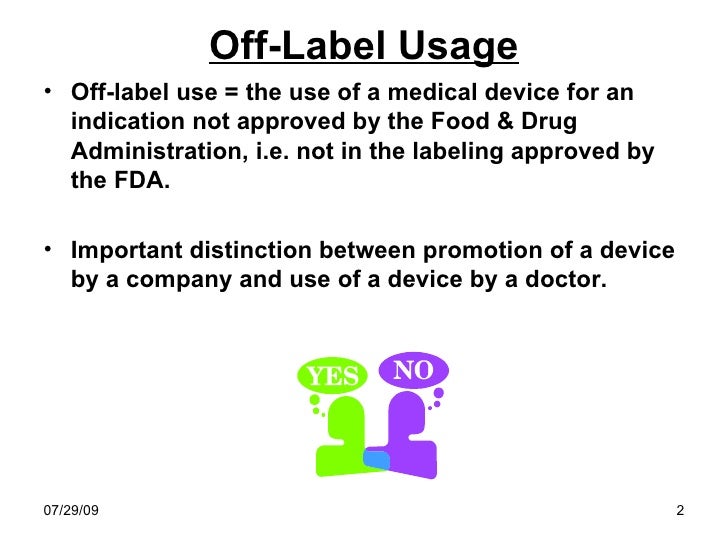
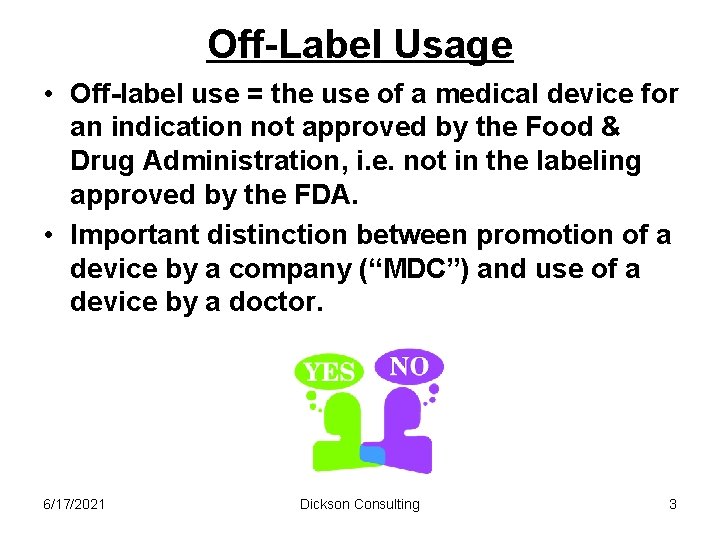
I. Off-Label Use is Important to the Proper Treatment of Patients. A treatment is "off-label" when the drug or device is used for another medical condition (progression of the illness or different illness) or patient type (gender or age), or is prescribed in a manner or dose different than the FDA approved. 3 Off-label use of treatments is lawful, and in many cases is critical to patient care.
Off label promotion medical device.
FDA releases new guidance on off-label promotion. Last week, the U.S. Food and Drug Administration ("FDA") released two guidance documents that relate to sharing of certain information about drugs and medical devices, including economic information about unapproved products and unapproved uses, as well as information not included in a ...
The term off-label drug use (OLDU) is used extensively in the medical literature, continuing medical education (CME) exercises, and the media. It is a polarizing term because it can be associated with great benefit or harm to patients.1 In addition, OLDU, along with allegations of pharmaceutical company promotion of OLDU, has been the cause of major lawsuits and historically large out-of-court ...
Off-Label Promotion of Medical Devices: Seeking Clues from the Past to Protect Against Increased Enforcement in the Future April 17, 2017 Amy Colvin and James R. Ravitz For Medical Device Pharmaceutical companies that promote their products for off-label use continue to be the subject of intense regulatory scrutiny. But they are no longer alone.
FDA defines off-label use of drugs as "use for indication, dosage form, dose regimen, population or other use parameter not mentioned in the approved labeling," and currently prohibits off-label promotion of drugs and medical devices. Off-label communication may mean many things, including manufacturer-physician communication, direct-to ...
FDA to clarify role of off-label uses in medical device approvals. September 23, 2020 By Nancy Crotti. The FDA has released proposed regulations to make clear that off-label use of a device alone will not be enough to sway the agency to give its blessing to that use. Regulators need more evidence, and the proposed guidance gives examples of ...
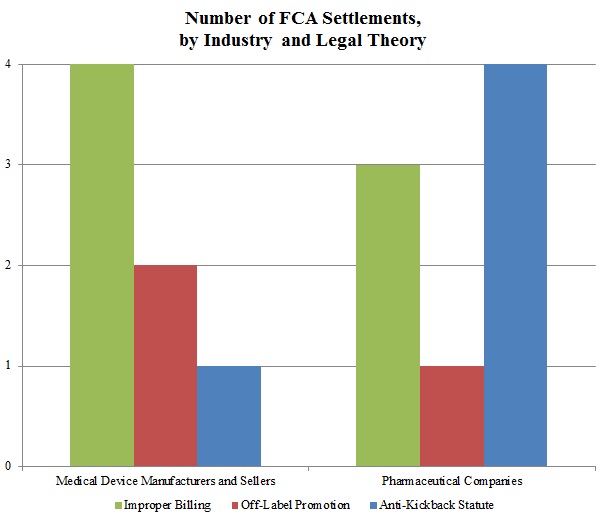
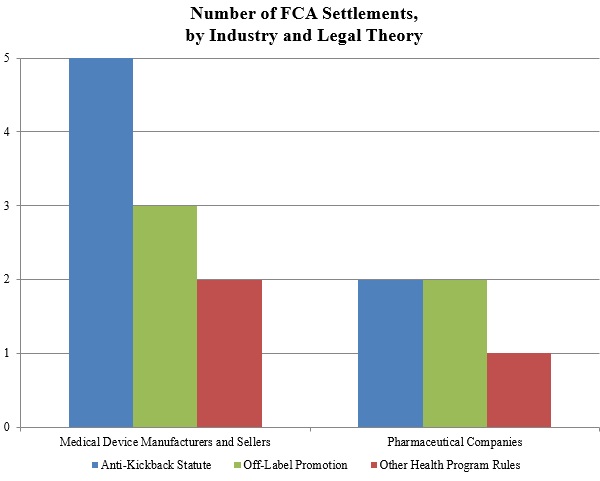
Off-Label Marketing and Promotion of Drugs and Medical Devices The largest fines levied under the False Claims Act by the Department of Justice have nearly all stemmed from unlawful off-label promotion of pharmaceutical products by the world's large pharmaceutical companies.
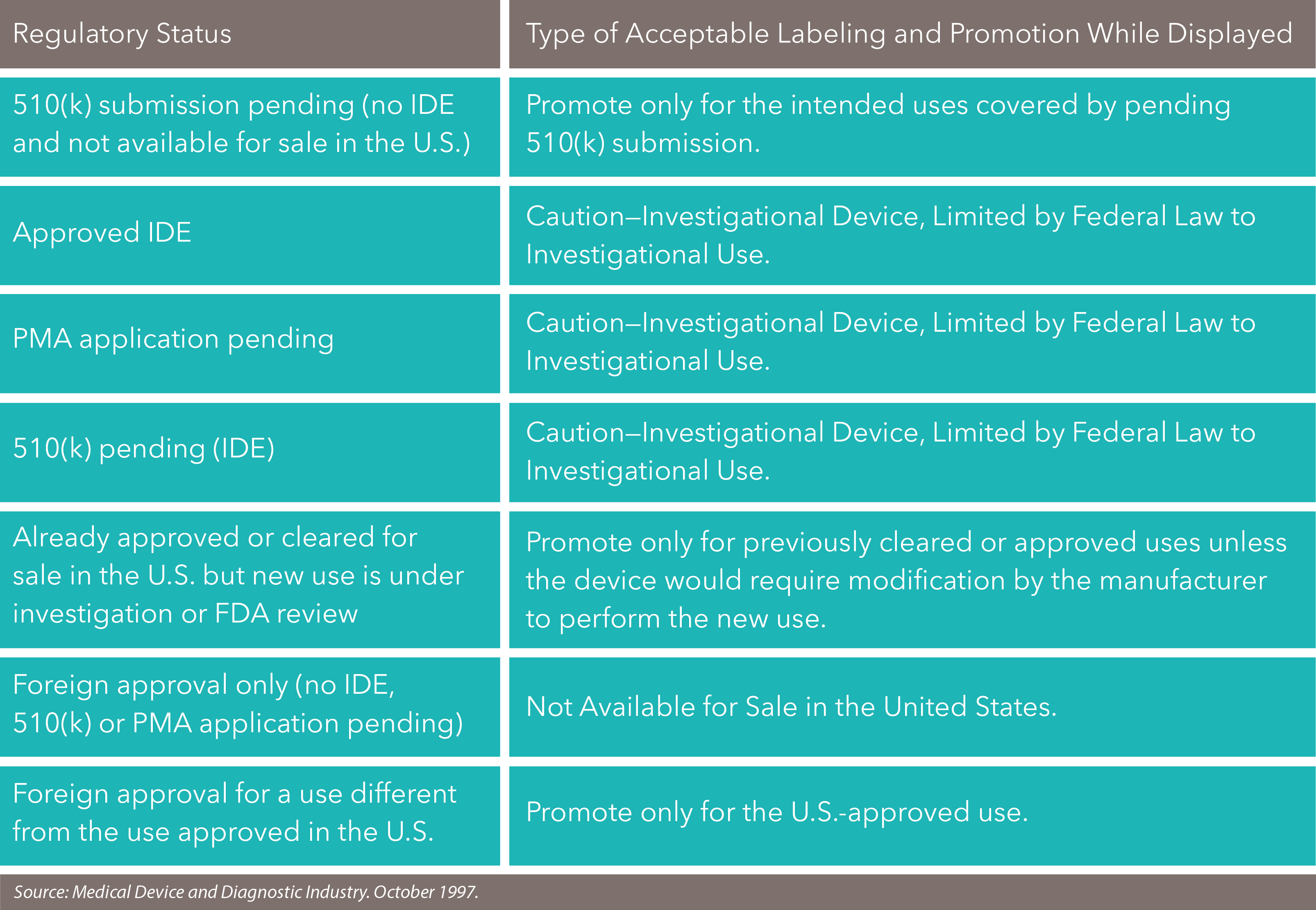
Devices that are not authorized for marketing include experimental (or investigational) devices as well as approved or cleared devices that are marketed for uses not on their approved or cleared labels ("off-label uses"). In both cases, the unauthorized intended use of the device would render the device "misbranded."
Promoting off-label use of a medical device or drug has landed companies in legal trouble. "Our final guidance now includes recommendations that are designed to enable truthful, non-misleading and appropriate company communications with insurers across a product's lifecycle," Gottlieb said.
employees may find useful with regard to off-label promotion of prescription drugs. This is not intended to be a complete list. U.S. Department of Health and Human Services ... Promotion of Unapproved Drugs and Medical Devices. Statement Before the Senate Committee on Labor and Human Resources. (1996, February 22). (Statement of William B ...
Federal law, however, prohibits the introduction of a drug or device into interstate commerce without approved labeling for intended uses, an act known as "misbranding," and the federal government has pursued enforcement actions for promoting drugs and devices for off-label uses. See 21 U.S.C. §§ 331 (a), 352 (a).
the fda has previously developed draft guidance - "responding to unsolicited requests for off-label information about prescription drugs and medical devices" that addresses how manufacturers can respond to unsolicited requests for off-label information relating to their fda-approved or cleared products without such responses being used as …
As a summary - any medical device, promotional or samples of medical devices to be "made available" on the EU market must be in conformity with the above regulatory frameworks - either on the device, the outer packaging or the IFUs. To know more about the promotion & advertising rules for medical devices, contact us
Federal law, however, prohibits the introduction of a drug or device into interstate commerce without approved labeling for intended uses, an act known as "misbranding," and the federal government...
Under the Federal Food, Drug, and Cosmetic Act, it is illegal for pharmaceutical companies to promote their products for uses not approved by the Food and Drug Administration (FDA), and corporations that market drugs for off-label indications may be subject to civil liability under the False Claims Act as well as criminal penalties. Contents
The FDA historically has held the view that off-label promotion of a device, or any medical product, is misbranding under the Food, Drug, & Cosmetics Act.
Off-label use of a device could lead to dangers including: adverse reactions inadequate sterilisation insufficient mechanical strength and/or structural integrity insufficient durability misuse due...
Module 3 : Off-Label Promotion of Medical Devices: Maximizing Your Performance Claims within FDA's Framework of Acceptable Practices. Areas Covered: What are the critical issues that arise in advertising and promotion of medical devices. What constitutes valid scientific evidence to support advertising and promotional claims.
FDA regulation of off-label promotion Under FDA rules, any promotional materials distributed by a company should be truthful, balanced, nonmisleading, and supported by substantial evidence. In...
Off-label drug or medical device "use" is the practice of prescribing drugs or medical devices to patients for a purpose not included on the federally approved label. Off-label "marketing" is the practice of attempting to influence physicians to prescribe drugs or devices for off-label purposes. The Federal Food and Drug
3 References 1. Ausness, R. (2008). “There’s Danger Here, Cherie!”: Liability for the Promotion and Marketing of Drugs and Medical Devices for Off-Label Uses.
May 06, 2020 · "Off-Label" and Investigational Use Of Marketed Drugs, Biologics, and Medical Devices Guidance for Institutional Review Boards and Clinical Investigators January 1998 Final Share
Implicit off-label promotion occurs when a manufacturer indirectly suggests that a device is appropriate for an unapproved use. For example, in 2000 a medical device company that manufactured a product cleared for use as an adjunctive diagnostic screening device for detecting breast cancer received a Warning Letter from FDA. The reason?




















0 Response to "36 off label promotion medical device"
Post a Comment