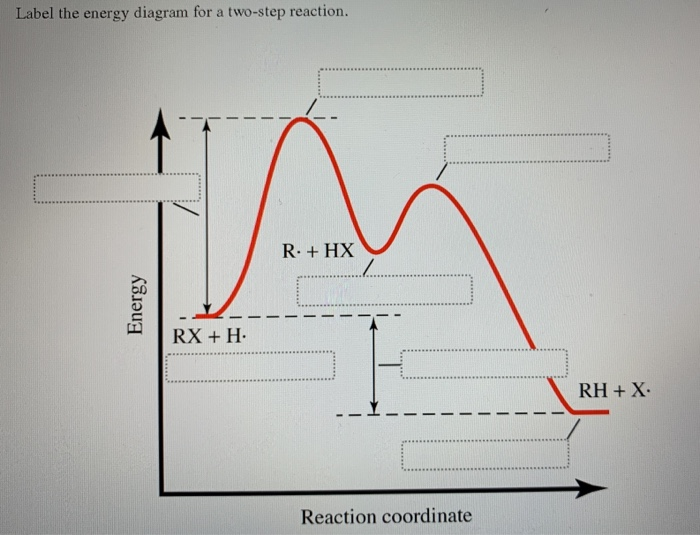
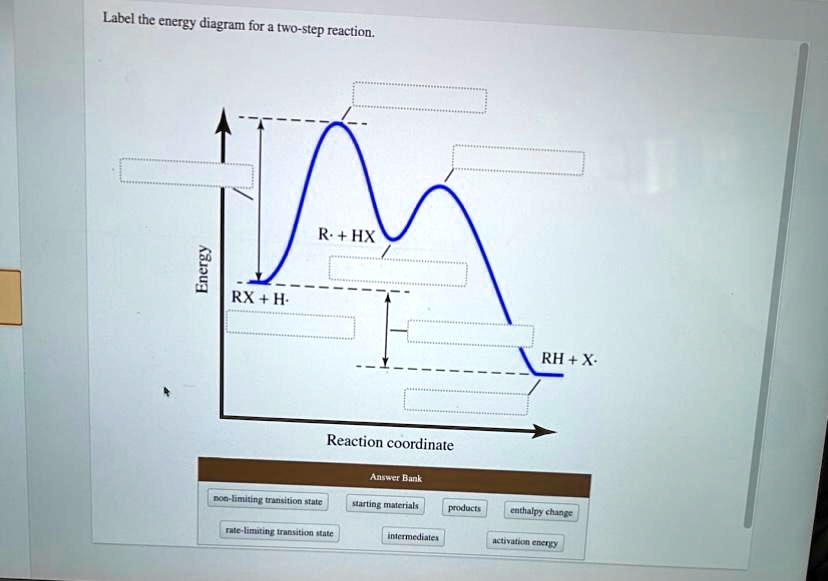
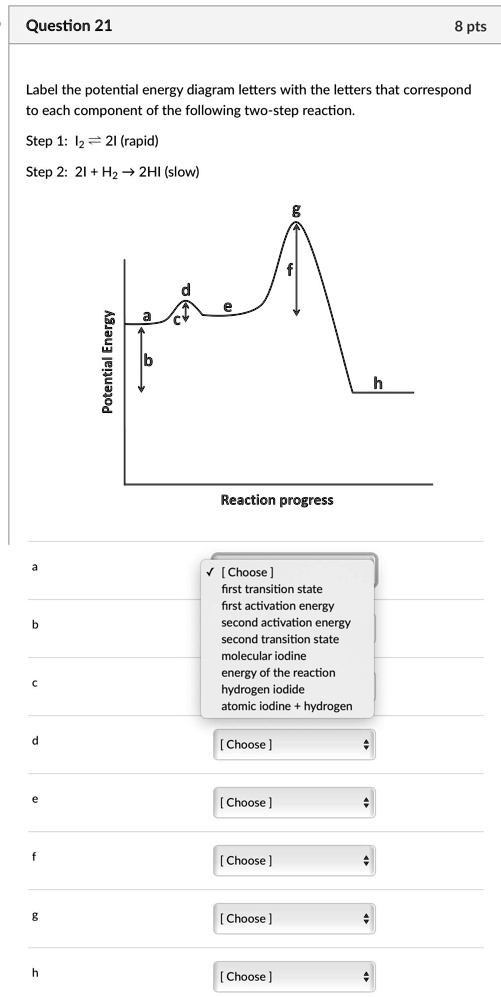
38 label the energy diagram for a two-step reaction
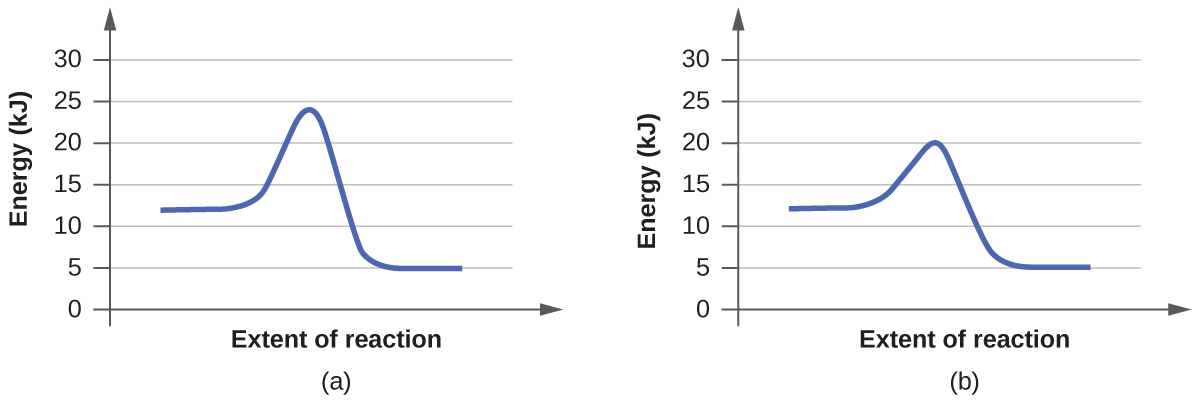
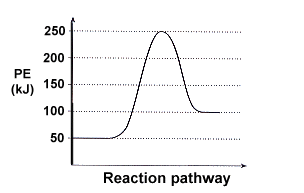
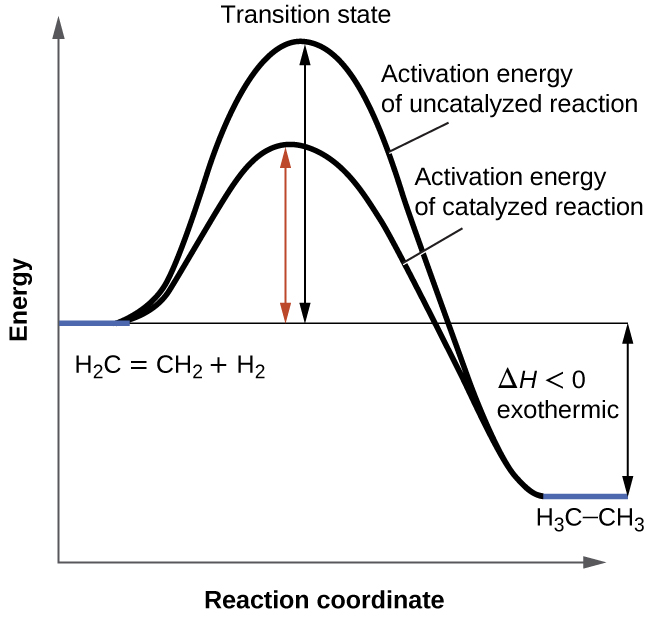
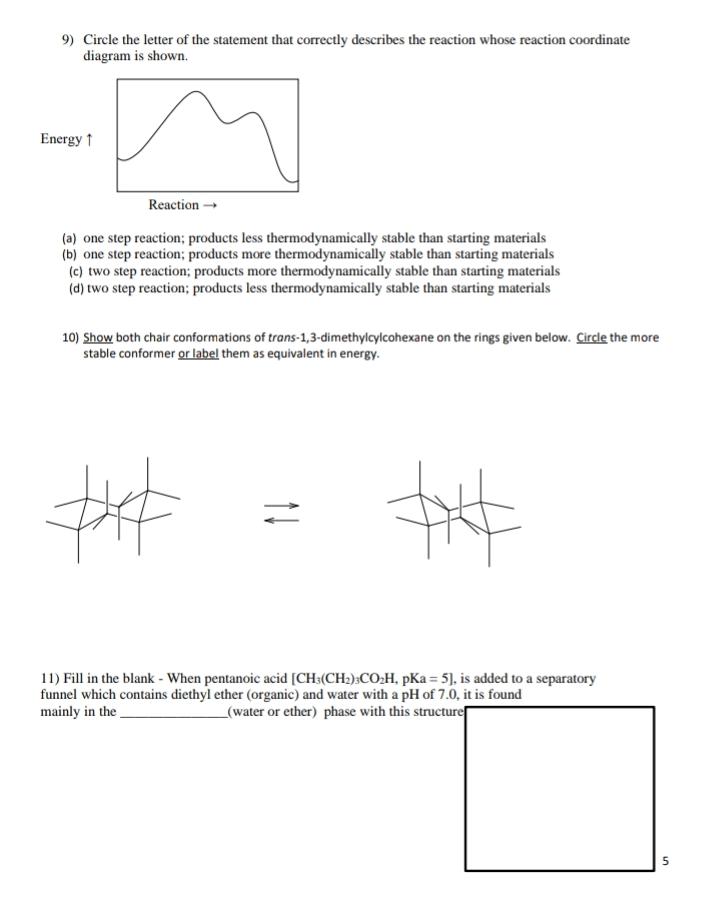
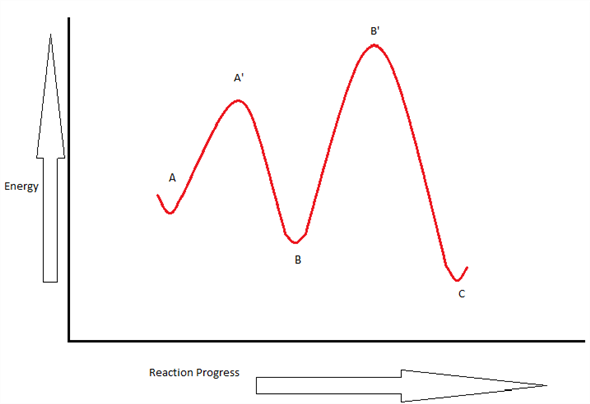
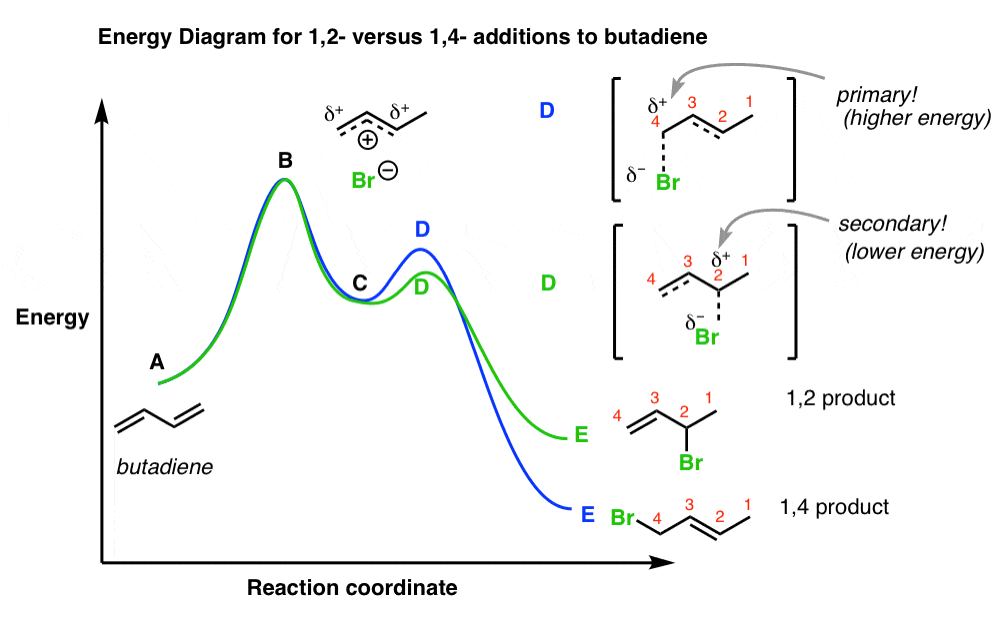
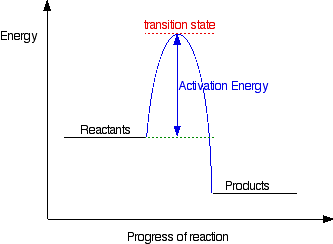
6.7: Energy Diagrams. You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ‘ reaction coordinate ’, tracing from left to right the progress of the reaction ... Which compound would you predict to be highest in energy? B. Calculate Ea for the conversion of C → B. +9 kcal. The following is an energy diagram for the conversion of A → B → C. The energies of activation and ∆H's for each step are also given. Calculate ∆H overall as shown on the energy diagram for A → B → C. +3 kcal.
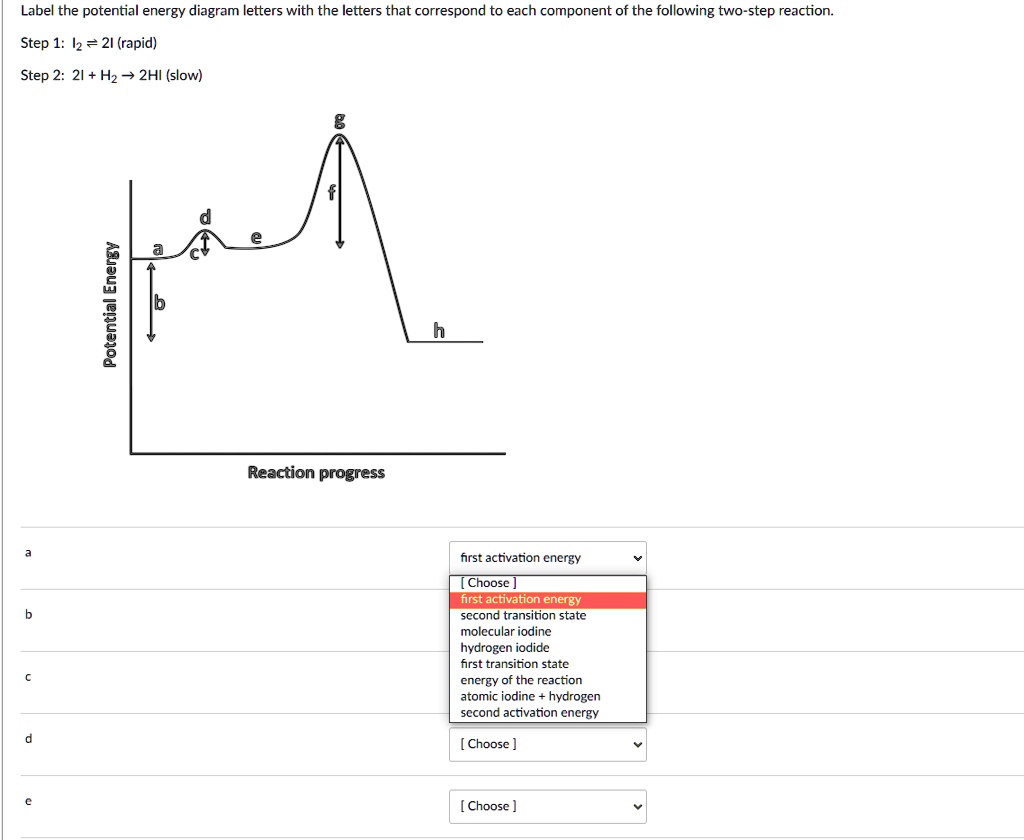
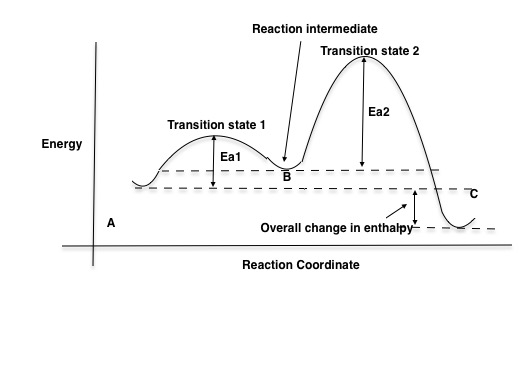
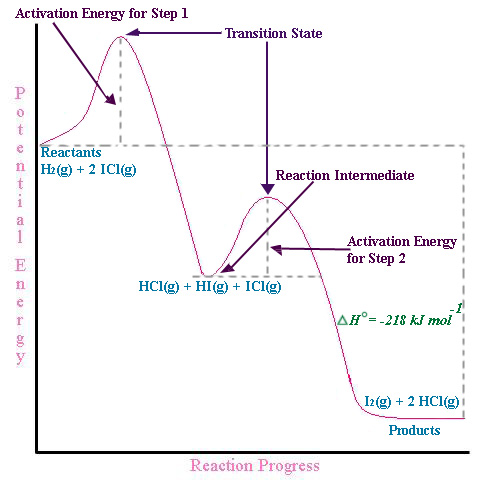
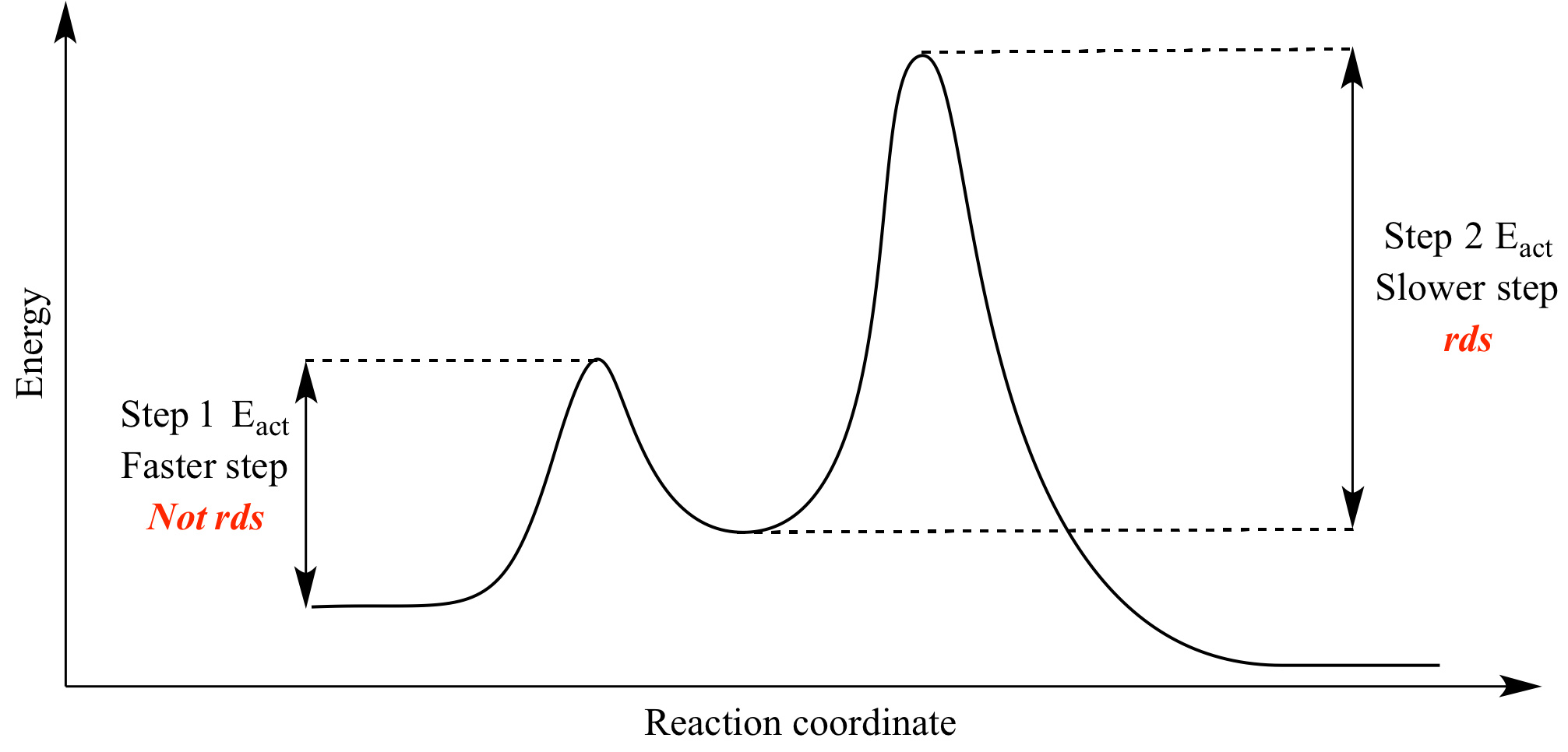
Oct 06, 2018 · The activation energy for each step is labeled e a1 and e a2. Label the energy diagram for a two step reaction. Endothermic because energy is needed to break the a b bond. Each elementary step has its own activated complex labeled ac 1 and ac 2.

Label the energy diagram for a two-step reaction
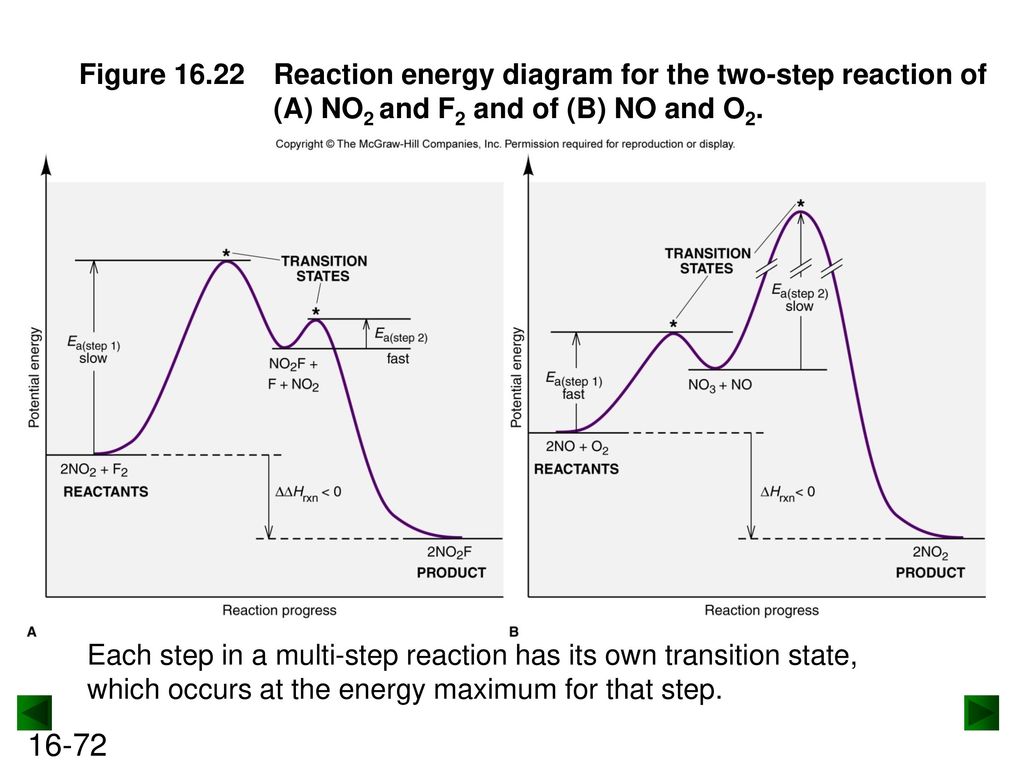
The reaction whose potential energy diagram is shown in the figure is a two-step reaction. The activation energy for each step is labeled \(E_{a1}\) and \(E_{a2}\). Each elementary step has its own activated complex, labeled \(AC_1\) and \(AC_2\). Note that the overall enthalpy change of the reaction \(\left( \Delta H \right)\) is unaffected by ... Label the energy diagram for a two-step reaction. Q. A reaction coordinate diagram is shown below for the reaction of A to form E. Answer the following questions.i) Identify the transition state (s)?ii) W... Q. Which reaction coordinate diagram represents a reaction in which the activation energy, Ea, is 50 kj.mol-1 and the ΔHrxn is -15 kj. mol-1? See the answer See the answer done loading. Show transcribed image text. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (42 ratings) Transcribed image text: Label the energy diagram for a two-step reaction.
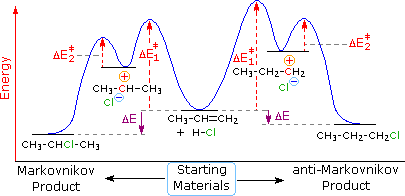
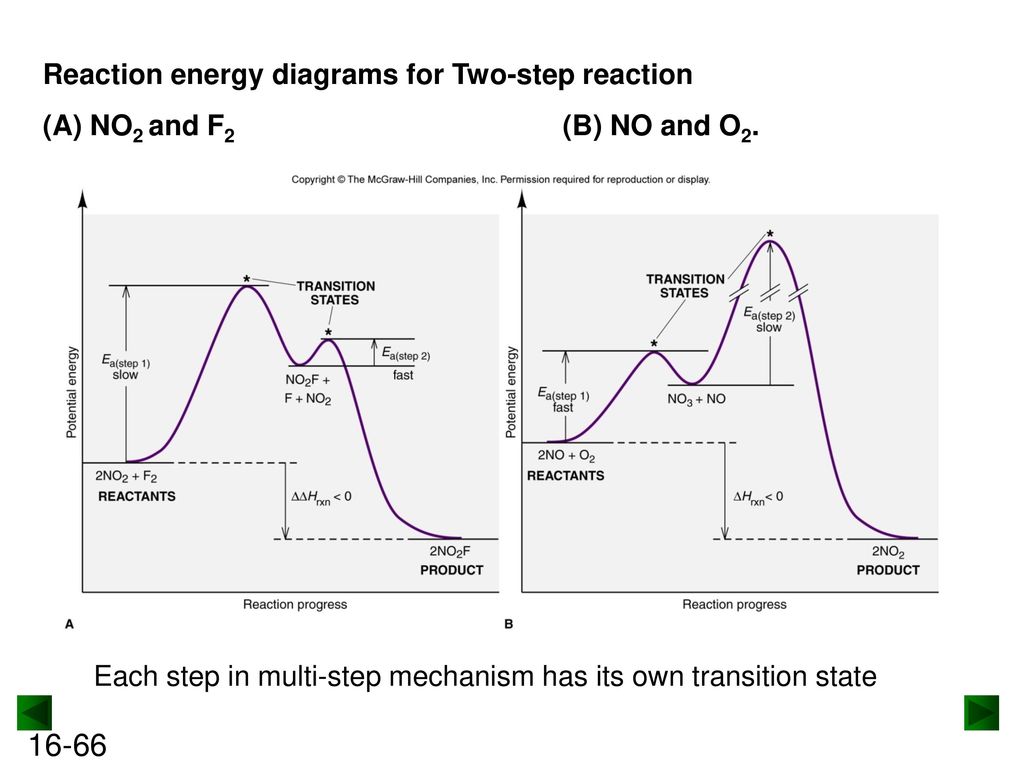
Label the energy diagram for a two-step reaction. We’re being asked to label the given energy diagram. Recall that an energy diagram is usually read from left to right. The components of a two-step energy diagram are: • Reactants: are placed on the left/beginning of the energy diagram • Products: are placed on the right/end of the energy diagram • Non-limiting transition state: is the transition state with the lowest energy in the energy diagram Organic Chemistry (13th Edition) Edit edition Solutions for Chapter 3 Problem 18P: Draw a reaction energy diagram for a two-step reaction that has an endothermic first step and an exothermic second step. Label the reactants, transition states, reaction intermediate, activation energies, and enthalpy differences. … A Two-Step Reaction Mechanism. The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and activation energy. The overall energy difference between the starting materials and products is delta H overall. Step 1 has the higher transition energy state, thus it is the rate-determining step. Sketch out an activation energy diagram for a multistep mechanism involving a rate-determining step, and relate this to the activation energy of the overall reaction. Write the rate law expression for a two-step mechanism in which the rate constants have significantly different magnitudes.
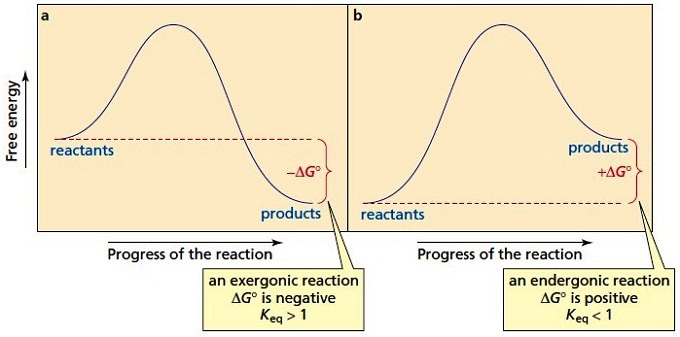
Alternate ISBN: 9781305638716, 9781305686465. Organic Chemistry (9th Edition) Edit edition Solutions for Chapter 6 Problem 21E: Draw an energy diagram for a two-step reaction with Keq > 1. Label the overall ΔG°, transition states, and intermediate. Is ΔG° positive or negative? …. See the answer See the answer done loading. Show transcribed image text. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (42 ratings) Transcribed image text: Label the energy diagram for a two-step reaction. Label the energy diagram for a two-step reaction. Q. A reaction coordinate diagram is shown below for the reaction of A to form E. Answer the following questions.i) Identify the transition state (s)?ii) W... Q. Which reaction coordinate diagram represents a reaction in which the activation energy, Ea, is 50 kj.mol-1 and the ΔHrxn is -15 kj. mol-1? The reaction whose potential energy diagram is shown in the figure is a two-step reaction. The activation energy for each step is labeled \(E_{a1}\) and \(E_{a2}\). Each elementary step has its own activated complex, labeled \(AC_1\) and \(AC_2\). Note that the overall enthalpy change of the reaction \(\left( \Delta H \right)\) is unaffected by ...









0 Response to "38 label the energy diagram for a two-step reaction"
Post a Comment