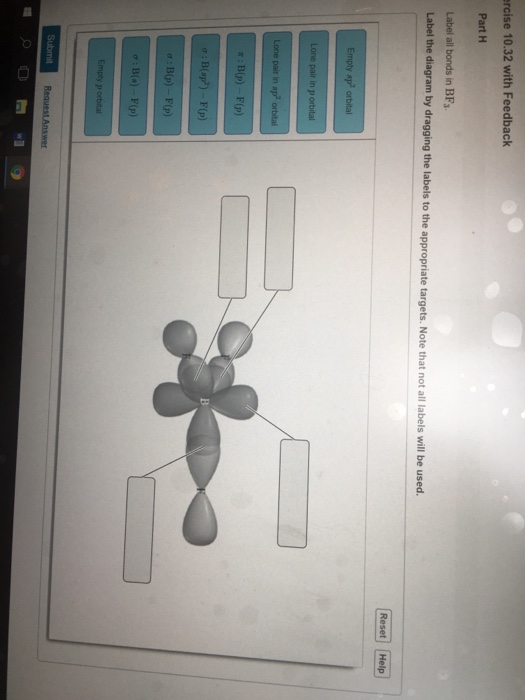
39 label all bonds in bf3
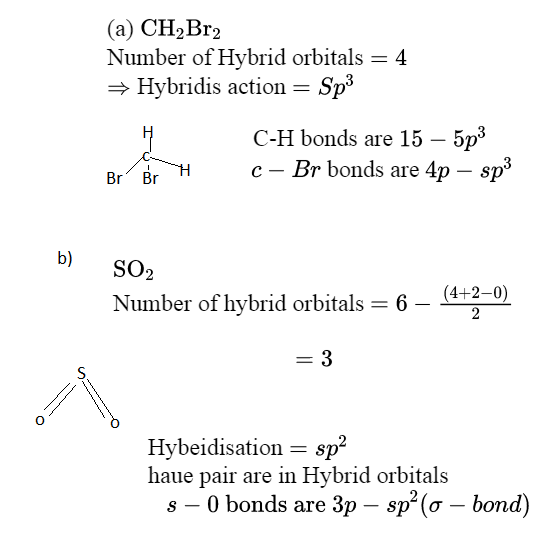
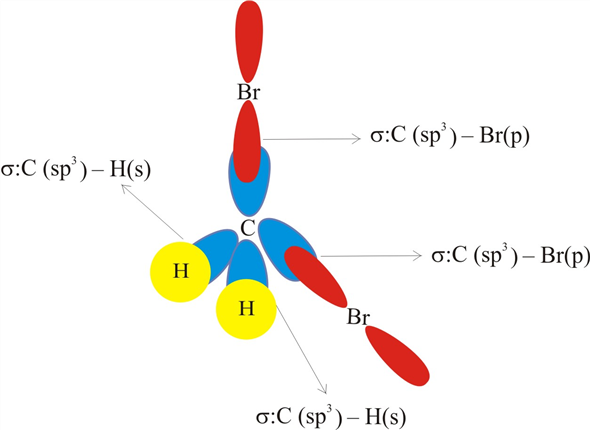
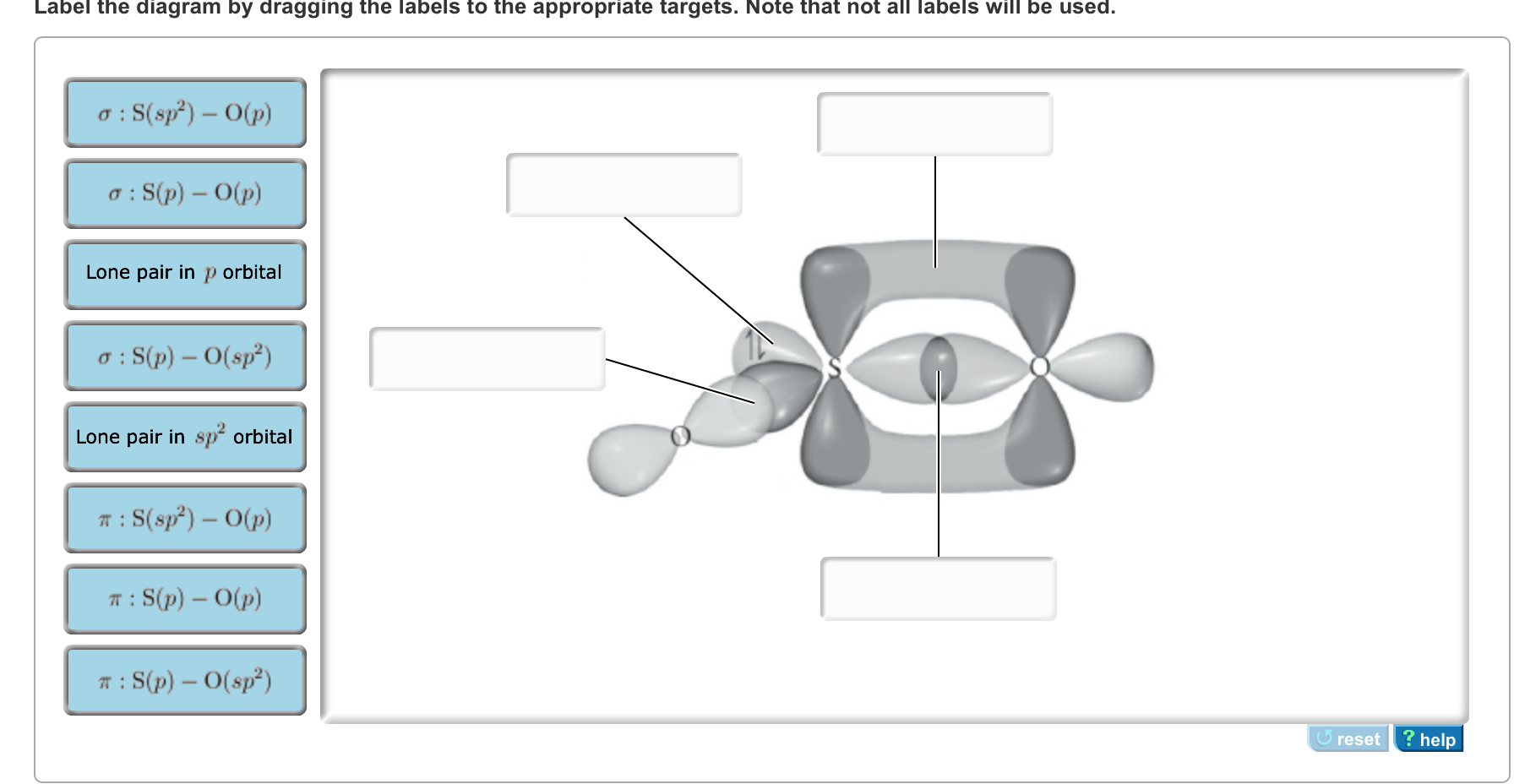
Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in NF3. Label all bonds in BF3. Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o C(sp) H(s) o C(sp') Br(s) C(p) H(p) C(p) Br(p) C(sp) H(p) o C(sps) Br (p) C(sps) Br (p) reset help Show transcribed image text Expert Answer . . . Show activity on this post. I'm trying to build a molecular orbital diagram for BF 3 and I'm running into problems with irreducible representations on the F side. 2s for B has an irreducible representation of A1. 2p for B has an irreducible representation of E' and A''2. 2s for F considered non bonding. 2p (along the bond axis) for F has an ...
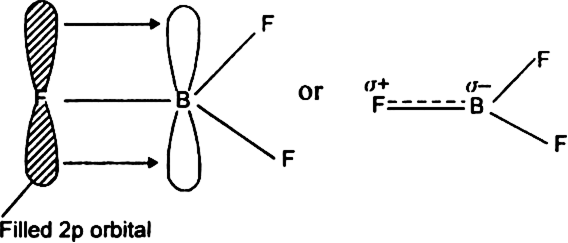
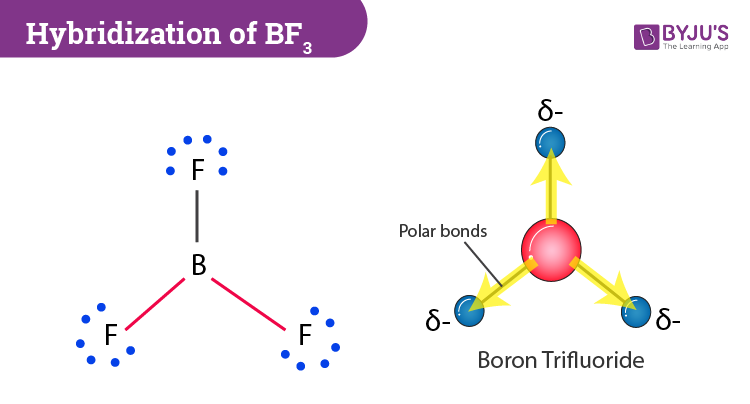
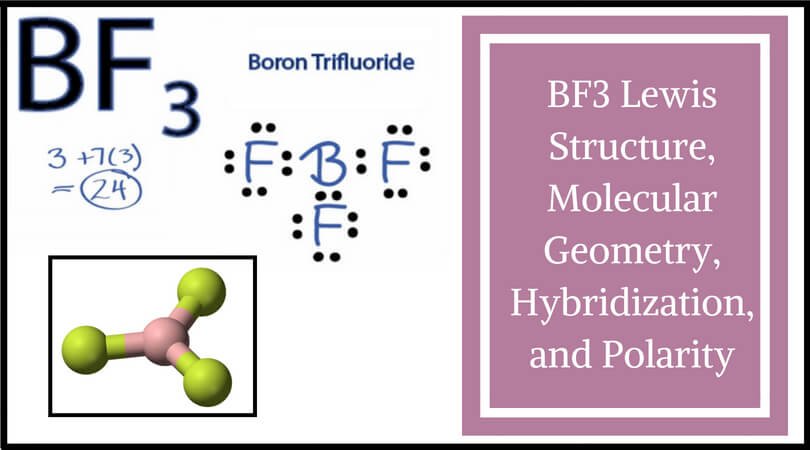
BF3 is SP2 hybridization. For this molecule, It is SP2 because one π (pi) bond is required for the double bond between the Boron and only three σ bonds are formed per Boron atom. The atomic S - orbitals and P - orbitals in Boron outer shell mix to form three equivalent SP2 hybrid orbitals.
Label all bonds in bf3
In the sketch of the structure of BF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Lone pair in sp? orbital T: B(p) - F(p) Empty p orbital Lone pair in p orbital B o : B(sp²) - F(p) o : B(s) - F(p) o : B(p) - F(p) Empty sp? orbital NH3 Lewis Structure, Geometry, and Hybridization. Ammonia is the simplest binary hydride made up of nitrogen and hydrogen denoted by its chemical formulae as NH3. It is a stable pnictogen hydride where all the atoms are covalently bonded to achieve a reactive state. Ammonia is lighter than the air, colorless, and pungent in smell. Bf3 Lewis Structure Molecular Geometry Hybridization And Polarity Mcat organic chemistry kaplan guide. Label all bonds in so2.. The so2 has a bond angle of 120 degree. So with three things around the sulfur it forms a trigonal planar geometry but since you cannot see the lone pair on the sulfur you say that the structure is bent. 0 lone pair in ...
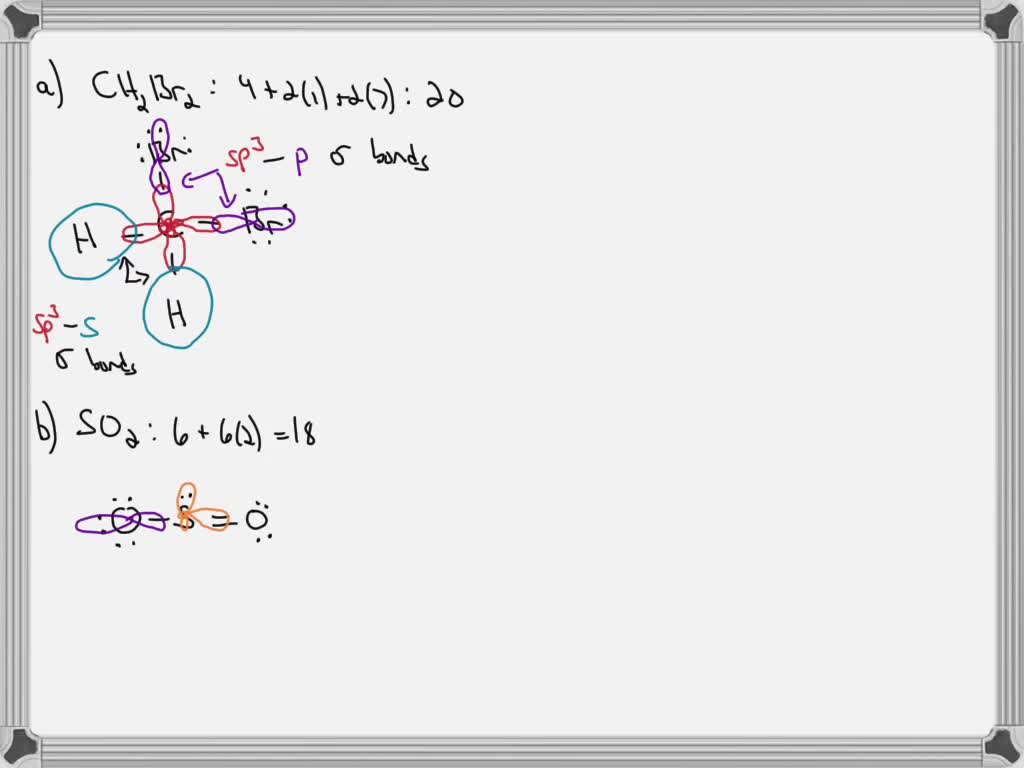
Label all bonds in bf3. The central atom carbon is bonded to two hydrogen atoms and two bromine atoms. All the bonds are sigma bonds. Write the electron configuration of the atoms as shown below. C (6): 1s22s22p2 Br (35): [Ar]3d104s24p5. As valency of carbon is 4, it can form maximum four bonds. Each of the hydrogen and bromine atoms can form a single bonds with ... 1) BF3. In BF3, three sp2 hybrid orbitals of boron form sigma bonds with three fluorine atoms. The three sp2 hybrid orbitals have a trigonal planar arrangement to minimize electron repulsion. 2) Propyne . In C3H4, both the 1 st and 2 nd carbon atoms are sp hybridised and the 3 rd carbon is sp3 hybridized. Label all bonds in bf3. Label each bond in the molecules as polar or nonpolar and give the shape of each molecule and describe whether each molecule and tell whether each is soluble or insoluble in water. The three sp2 hybrid orbitals have a trigonal planar arrangement to minimize electron repulsion. In bf3 three sp2 hybrid orbitals of boron ... Sulfur dioxide is spelled as Sulphur dioxide in Commonwealth English. This is a pungent-smelling, colorless gas. Talking about its properties, SO2 has a molar mass of 64.066 g/mol. The melting point and boiling points are -72℃, and -10℃ respectively. Now let's move on to the fundamental concepts like lewis structure, molecular geometry ...
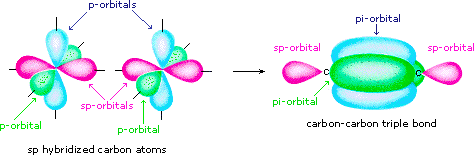
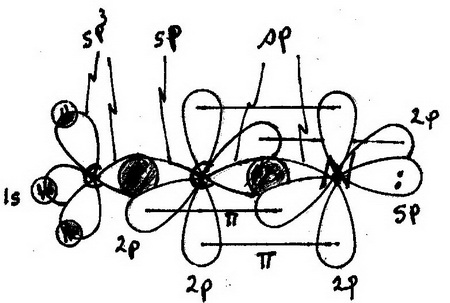
Answer (1 of 15): sp2 hybridisation Electronic configuration of boron is (1s)2 (2s)2 (2px)1 (2py)0 (2pz)0. Valence orbitals for boron are 2s, 2px, 2py and 2pz. As we ... of a π-bond is concentrated above and below a plane containing the bonded atoms and arises from overlap of two p-orbitals pointing in the same direction. So, a double bond contains 1σ + 1π bond and a triple bond contains 1σ + 2π bonds. e.g. CH 4 and C2H6 contain all σ-bonds. Ethylene, C2H4 has the Lewis Structure: Solution for What is the Lewis Structure for BF3, the steric number, the electron pair geometry, ... draw and label FUNCTIONAL groups Answer all questions. A: ... We may draw bond vectors for each individual bond, and then say if the resultant dipole moment vecto ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 86% (7 ratings) Transcribed image text: Label all bonds in BF_3. Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used.
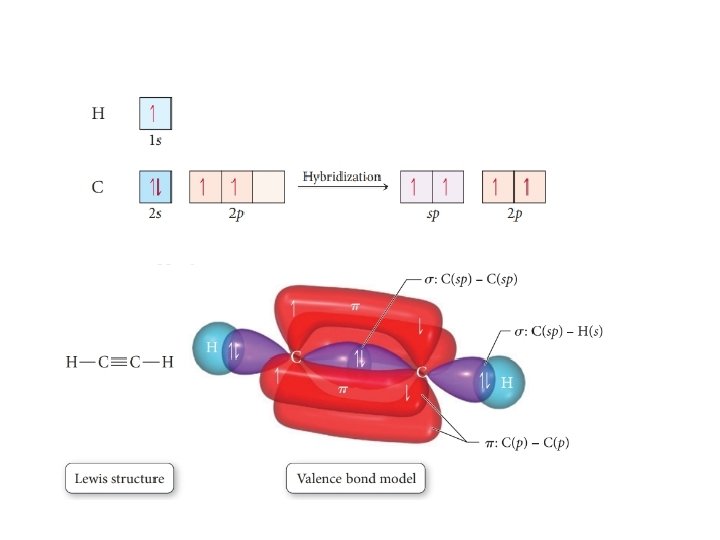
Valence Bond Theory 62. Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. CH2Br2 b. SO2 c. NF3 d. BF3 SO, NOT ALL bonds in a molecule are capable of absorbing IR- energy. Infrared Absorption For a molecule to show infrared absorptions it must possess a specific feature: an electric dipole moment which must change during the vibration. A dipole moment, µ is defined as the charge value (q) multiplied ... All the bonds in BF 3 are sigma bonds. BF 3 Molecular Geometry and Bond Angles. Normally, boron forms monomeric covalent halides which have a planar triangular geometry. This shape is mainly formed by the overlap of the orbitals between the two compounds. To be more precise, the BF 3 molecular geometry is trigonal planar. It further has ... We are asked to label all the bonds in SO 2. Step 1: Sulfur (EN = 2.5) is less electronegative than oxygen (EN = 3.5) so sulfur is the central atom. Step 2: The total number of valence electrons present in SO 2 is: Group Valence Electrons. S 6A 1 × 6 e - = 6 e -
Label all bonds in so label the diagram by dragging the labels to the appropriate targets. Label all bonds using the σ or π notation followed by the type of overlapping orbitals. The hybridization of sulfur atom is sp2 hence a lone pair and two bond pairsdue to sigma bonding reside in these hybrid orbitals.
carbon sigma bond is formed from overlap of an sp3 hybrid orbital on each C atom. Ethanol, C2H6O has 2(4) + 6(1) + 6 = 20 e − The two C atoms and the O atom are sp3 hybridized. All bonds are formed from overlap with these sp3 hybrid orbitals. The C‒H and O‒H sigma bonds are formed from overlap of sp3 hy-brid orbitals with hydrogen 1s ...
Label all bonds in bf3. Label all bonds in bf3. Label each bond in the molecules as polar or nonpolar and give the shape of each molecule and describe whether each molecule and tell whether each is soluble or insoluble in water. The three sp2 hybrid orbitals have a trigonal planar arrangement to minimize electron repulsion.
Boron is the least electronegative atom in the BF 3 Lewis structure and therefore goes at the center of the structure. Boron is an exception and only needs 6 valence electrons in its outer shell. If we check the formal charges for the BF 3 Lewis structure we will find that they are zero even though B only had six valence electrons.
FREE Expert Solution. We are asked to label all the bonds in NF3. Step 1: Determine the central atom in this molecule. • N→ less electronegative atom than F → central atom. Step 2: Calculate the total number of valence electrons present. NF3: Group Valence Electrons. N 5A 1 × 5 e- = 5 e-. F 7A 3 × 7 e- = 21 e-.
Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. CH2Br2 b. SO2 c. NF3 d. BF3
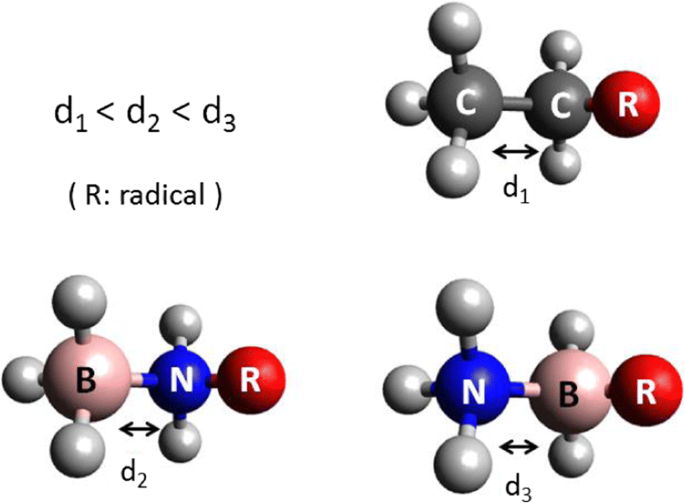
Warning! Long Answer. Here's what I get. > Step 1. Draw the Lewis structure (a) Start with a skeleton structure. The two "C" atoms (least electronegative) will be the central atoms, with the "N" attached to one of the carbons. (b) Attach the hydrogen atoms. The question gives you a clue where they go. The formula "NCCH"_3 tells you that the three "H" atoms are attached to the terminal carbon atom.
Boron trifluoride is a colorless gas with a pungent odor. It is toxic by inhalation. It is soluble in water and slowly hydrolyzed by cold water to give off hydrofluoric acid, a corrosive material.Its vapors are heavier than air. Prolonged exposure of the containers to fire or heat may result in their violent rupturing and rocketing.
Label all bonds in BF3. Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. σ : B(s)-F(p) Empty p orbital Lone pair in sp orbital σ : B(sp2)-F(p) σ : B(p)-F(p) Lone pair in p orbital Empty sp2 orbital π : B(p)-F(p) Question: Label all bonds in BF3. Label the diagram by dragging the ...
Label all bonds in ch2br2.. It is a member of bromomethanes and a bromohydrocarbon. Label all bonds in ch 2 br 2. It has a role as a marine metabolite and an algal metabolite. Note that not all labels will be used. Write a hybridization and bonding scheme for each molecule. Label all bonds in ch2br2 label all bonds in ch2br2 elegant chemistry ...
Draw The Lewis Structure Of Nf3, NF3 Lewis Structure How to Draw the Dot Structure for, Solved: Draw the Lewis structure for NF3 What are its, Draw The Lewis Structure For Nf3, Draw The Lewis Structure For Nf3
Bf3 Lewis Structure Molecular Geometry Hybridization And Polarity Mcat organic chemistry kaplan guide. Label all bonds in so2.. The so2 has a bond angle of 120 degree. So with three things around the sulfur it forms a trigonal planar geometry but since you cannot see the lone pair on the sulfur you say that the structure is bent. 0 lone pair in ...
NH3 Lewis Structure, Geometry, and Hybridization. Ammonia is the simplest binary hydride made up of nitrogen and hydrogen denoted by its chemical formulae as NH3. It is a stable pnictogen hydride where all the atoms are covalently bonded to achieve a reactive state. Ammonia is lighter than the air, colorless, and pungent in smell.
In the sketch of the structure of BF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Lone pair in sp? orbital T: B(p) - F(p) Empty p orbital Lone pair in p orbital B o : B(sp²) - F(p) o : B(s) - F(p) o : B(p) - F(p) Empty sp? orbital











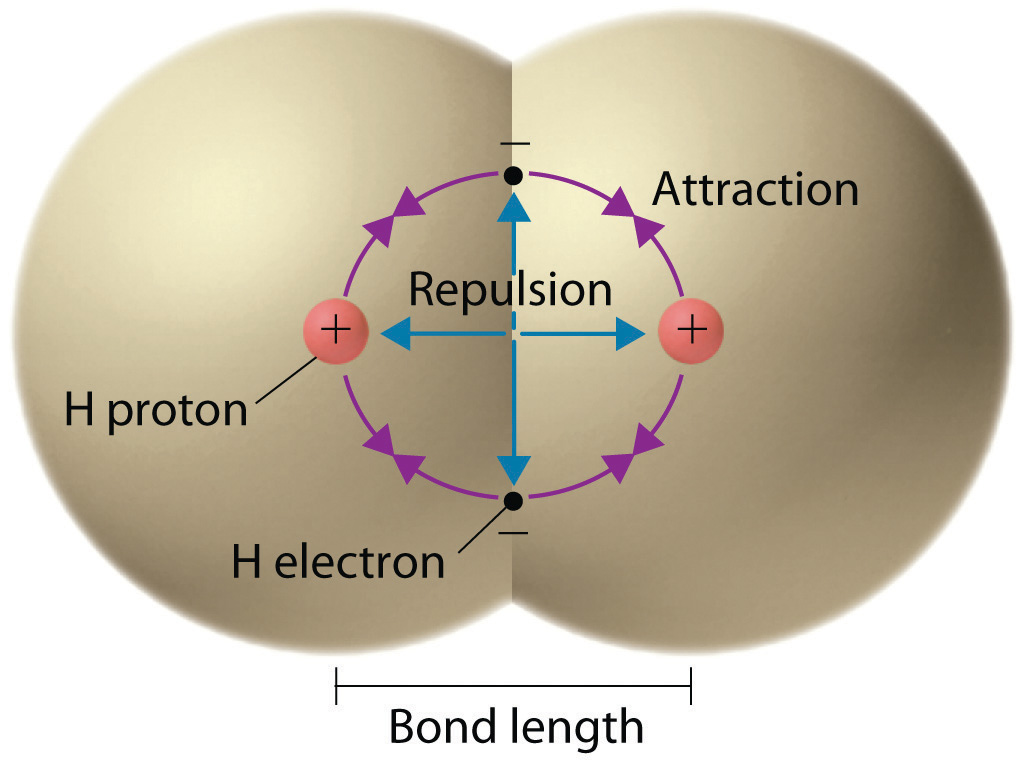



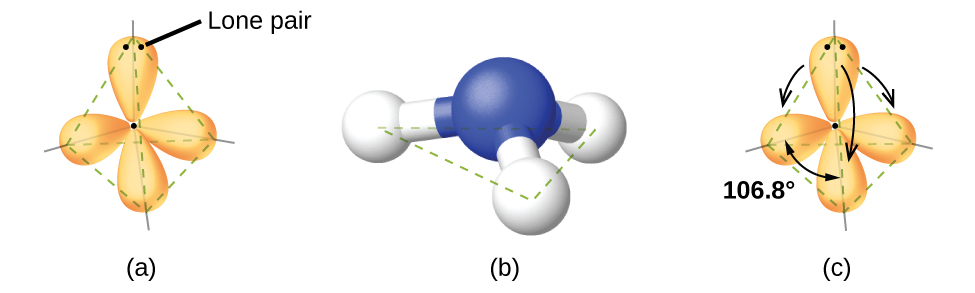
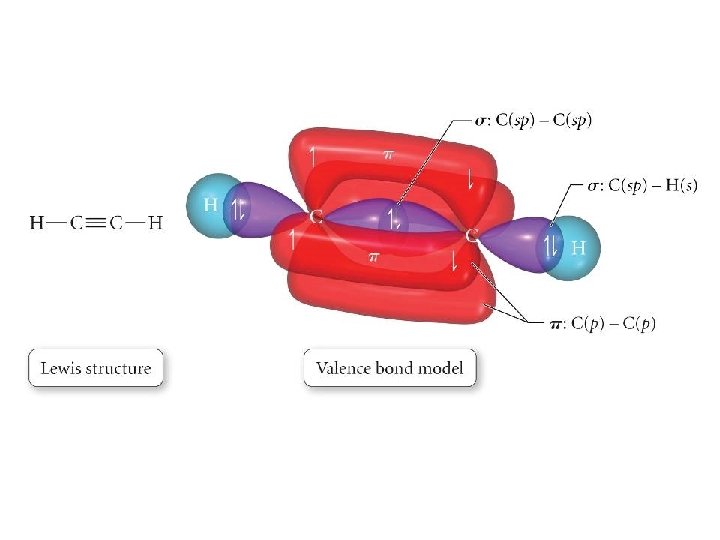


0 Response to "39 label all bonds in bf3"
Post a Comment