36 open-label study
Kelley JM, Kaptchuk TJ, Cusin C, Lipkin S, Fava M. Open-label placebo for major depressive disorder: a pilot randomized controlled trial. Psychother Psychosom. 2012;81(5):312-4. Article PubMed Google Scholar 21. Sandler AD, Bodfish JW. Open-label use of placebos in the treatment of ADHD: a pilot study. Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population.
report the long-term results of the CheckMate 204, an open-label, multicentre, phase 2 study assessing the efficacy of the combination of nivolumab plus ipilimumab in patients with active melanoma brain metastases. Interestingly, these results show that the objective intracranial response to nivolumab plus ipilimumab might be similar to the ...

Open-label study
This is an open-label, prospective study which evaluates the response of 4 teaspoons of Poly MVA taken daily, over a 24-Week interval. Study Design Go to Resource links provided by the National Library of Medicine MedlinePlus Genetics related topics: Amyotrophic lateral sclerosis Juvenile primary lateral sclerosis If undertaken primarily to gather more patient-years of exposure to the new drug in order to understand and gain confidence in its safety profile, open-label extension studies can play a useful and legitimate role in drug development and therapeutics. Open-label extension studies: In these studies, which often follow a double-blind randomized placebo-controlled trial, subjects have the option of remaining on the study intervention in an open-label fashion (i.e., they know that they are on the study intervention) for an extended period of time (e.g., several years).
Open-label study. In this phase 3, open-label study, patients with CML-CP previously treated with ≥2 TKIs were randomized (2:1) to receive asciminib 40 mg twice daily vs bosutinib 500 mg once daily. Randomization was stratified by major cytogenetic response (MCyR) status at baseline. The open-label study, sponsored by Johnson and Johnson Pharmaceutical Research and Development, included 204 children with confirmed acute otitis media who received at least one 10-mg/kg dose of the study medication (Pediatr. Levofloxacin succeeds vs. AOM This study is an ongoing, phase 2, multicentre, open-label, descriptive trial at six clinical research sites in the USA. The study was initiated on July 16, 2021, and has a planned completion date of Feb 15, 2022. Studies suggest that an open-label placebo (OLP) approach—in which patients are aware that they are receiving a placebo—can benefit adults, but little is known about this in children.
This is a single-arm, open-label feasibility study. A maximum of 15 participants will be enrolled. All participants will undergo a novel neurofeedback intervention, targeting down-regulation of deep limbic structures, specifically the amygdalae. Guidance for Clinical Trial Sponsors . Establishment and Operation of Clinical Trial Data Monitoring Committees . For questions on the content of this guidance, contact the Office of Communication ... This study is an ongoing, phase 2, multicentre, open-label, descriptive trial at six clinical research sites in the USA. The study was initiated on July 16, 2021, and has a planned completion date of Feb 15, 2022. Open Label Study to Evaluate the Efficacy of the Home Based Electrical Transcutaneous URIS I in the Treatment of Overactive Bladder (OAB) in Subjects Who Failed Any Pharmacotherapy. The safety and scientific validity of this study is the responsibility of the study sponsor and investigators.
Open-label trials may be performed to study the effectiveness of similar medications. Participants in a clinical trial are provided with a great deal of information about the trial design as well as the risks and benefits of their participation. This is designed to allow people to make an informed choice about their participation. In the present prospective, randomized, controlled, and open-label study, a daily dose of 1000 mg of QP was investigated for 30 days in 152 COVID-19 outpatients to disclose its adjuvant effect in treating the early symptoms and in preventing the severe outcomes of the disease. Results Open label trials are sometimes referred to as "non-masked" or "unblinded." If the trial is a non-pharmacological study, such as a trial of devices, or psychological and physical treatments, it may be referred to simply as "open." After recruitment to the trial, the participants were allocated to treatment using block randomisation. For example, in an open-label extension study of etanercept for ankylosing spondylitis, "efficacy analyses are reported without statistical inference between treatment groups"3. Although the CONSORT guideline has provided a frame-work for the design, analysis, and reporting of RCT4, no consensus statement has been developed for open-label
An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. In particular, both the researchers and participants know which treatment is being administered.
Patients were treated for 52 weeks in an open-label manner. Results: Canagliflozin significantly reduced hemoglobin A1c, fasting plasma glucose and bodyweight in all the study groups. Improvements were apparent by 4 weeks of treatment, and were maintained for 52 weeks.
This investigator-initiated feasibility study was conducted by Maryland Oncology Hematology at the Aquilino Cancer Center in Rockville, Maryland, USA. It was an open-label study involving 30 patients with cancer diagnosis and major depressive disorder (MDD), all of whom completed the study.
Open label extension studies are a common adjunct to double blind randomised controlled trials of new drugs The aim of open label extension studies often seems to be to enable continued use of a new drug for marketing or compassionate purposes rather than to increase knowledge
TAPPS was an open-label trial whose primary outcome was whether the patient was referred for a pleural drainage procedure. Allowing a blinded assessor to decide whether to refer the patient for a procedure was not feasible as many clinicians may be reluctant to enrol patients into the trial if they cannot be involved in their care during follow-up.
The goal of this study is to determine at what dose the study treatment (ASP9801) is safe and tolerated in study participants with cancer who have tumors that cannot be removed (unresectable) or have A Phase 1 Open-label Study of ASP9801, an Oncolytic Virus, Administered by Intratumoral (IT) Injection in Patients with Advanced/Metastatic Solid ...
NCI's Dictionary of Cancer Terms provides easy-to-understand definitions for words and phrases related to cancer and medicine.
Open-label: A term used to describe the situation when both the researcher and the participant in a research study know the treatment the participant is receiving. Open-label is the opposite of double-blind when neither the researcher nor the participant knows what treatment the participant is receiving.
an open label study. METHODS. We propose two methods to blind the data elements that can reveal the treatment assignment information in an open label study. After blinding the data, the sponsor personnel (such as physicians and statisticians) can access the blinded data.
The Phase 1/2 open-label trial is designed to assess the safety and efficacy of a single dose of CTX001 in patients ages 18 to 35 with TDT, non-beta zero/beta zero subtypes. Vertex Pharmaceuticals (NASDAQ: VRTX) - CRISPR Therapeutics and Vertex Announce FDA Fast Track Designation for CTX001 for the Treatment of Beta Thalassemia -- 16/4/2019
Open-label extension studies: In these studies, which often follow a double-blind randomized placebo-controlled trial, subjects have the option of remaining on the study intervention in an open-label fashion (i.e., they know that they are on the study intervention) for an extended period of time (e.g., several years).
If undertaken primarily to gather more patient-years of exposure to the new drug in order to understand and gain confidence in its safety profile, open-label extension studies can play a useful and legitimate role in drug development and therapeutics.
This is an open-label, prospective study which evaluates the response of 4 teaspoons of Poly MVA taken daily, over a 24-Week interval. Study Design Go to Resource links provided by the National Library of Medicine MedlinePlus Genetics related topics: Amyotrophic lateral sclerosis Juvenile primary lateral sclerosis


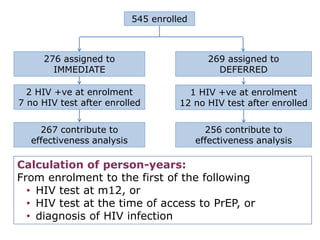



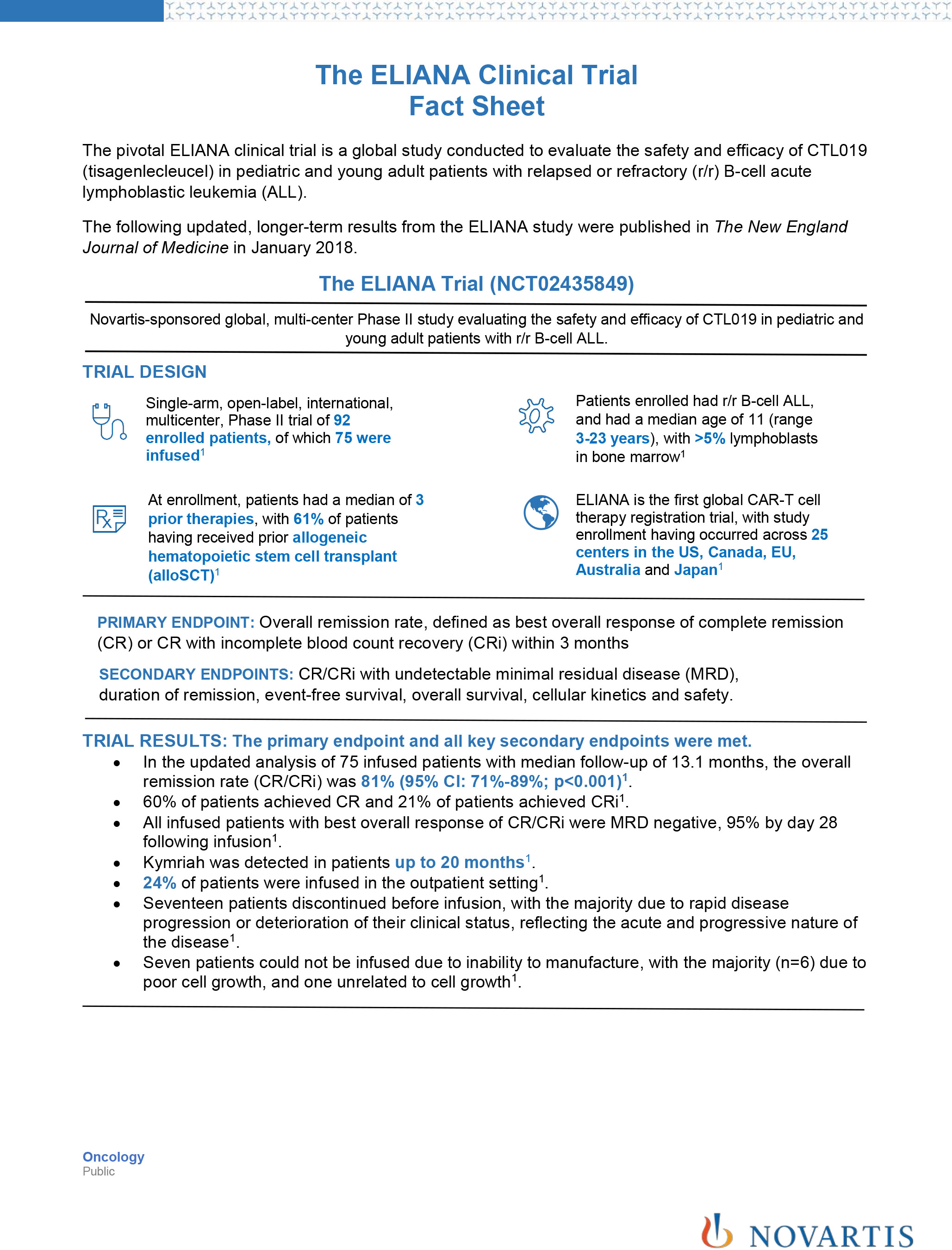
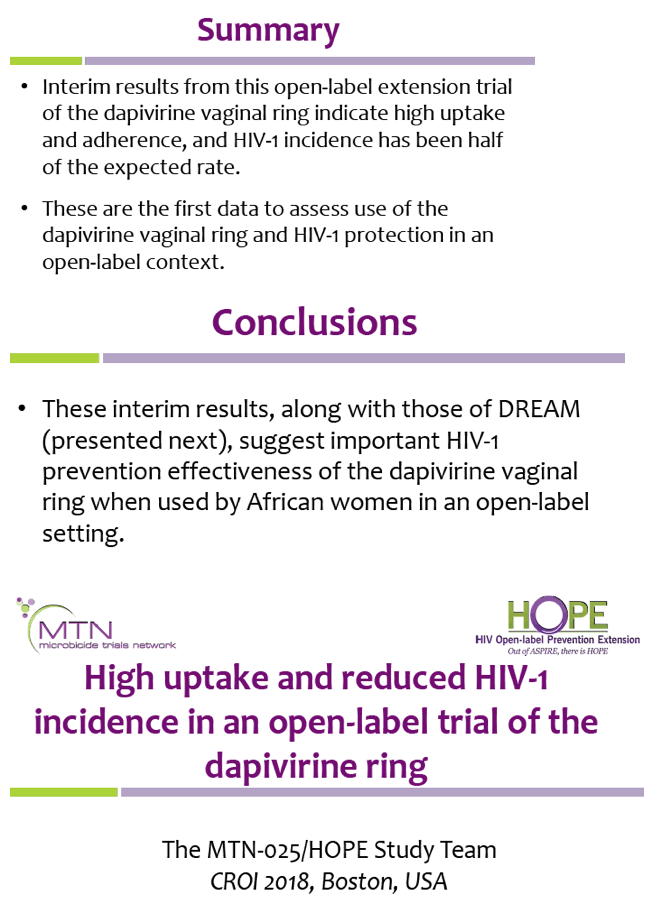
![PDF] Bias was reduced in an open-label trial through the ...](https://d3i71xaburhd42.cloudfront.net/814ad6ccbfa9defca4d2b00c4672f9070cf6b8da/16-Figure1-1.png)



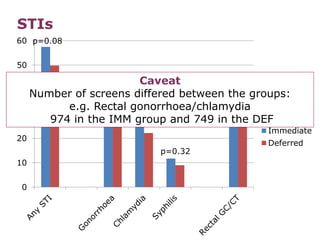


![PDF] Open-label versus double-blind placebo treatment in ...](https://d3i71xaburhd42.cloudfront.net/60afa7902fddea770036d6744ee84027545bd238/3-Figure1-1.png)
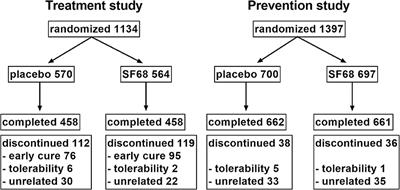










![PDF] An open-label study to evaluate the long-term safety and ...](https://d3i71xaburhd42.cloudfront.net/7f398e06a187a1dfceae4e18656100de18a56b28/5-Figure1-1.png)

0 Response to "36 open-label study"
Post a Comment