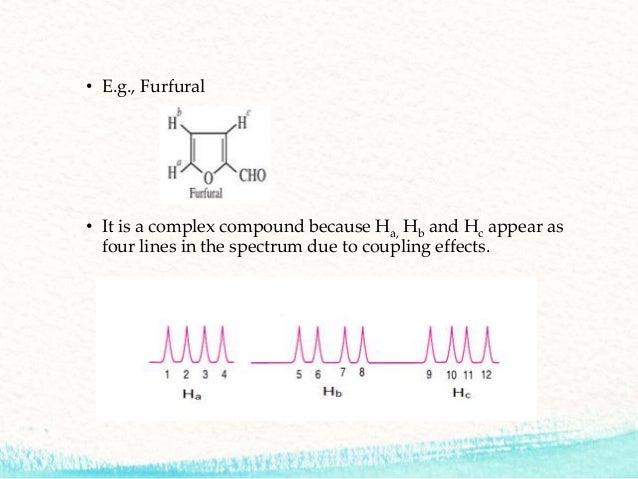
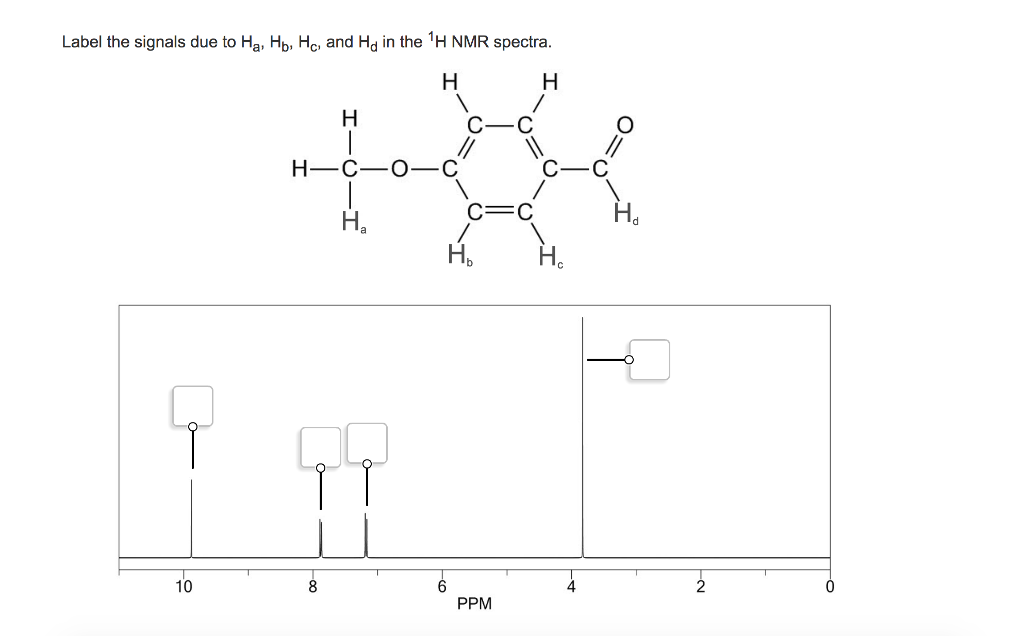
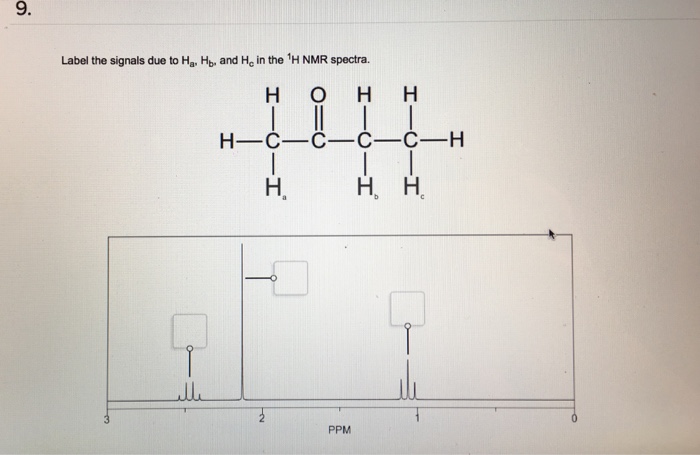
38 label the signals due to ha, hb, and hc in the 1h nmr spectra.
_____ is commonly used as an internal reference in NMR spectroscopy; its signal is assigned d = 0 in 1H and 13C NMR spectroscopy. Tetramethylsilane, (CH3)4Si On a 90 MHz spectrometer, calculate the frequency at which a proton absorbs if it appears at 4.20 ppm. Deciphering 1H NMR Spectra. One of the most important concepts taught in organic chemistry is the method for determining the chemical structure of newly synthesized or unknown compounds. In this article, we will summarize the concept of proton NMR, the most common NMR information acquired by organic chemists.
The isomeric pairs previously cited as giving very similar proton nmr spectra are now seen to be distinguished by carbon nmr. In the example on the left below (blue box), cyclohexane and 2,3-dimethyl-2-butene both give a single sharp resonance signal in the proton nmr spectrum (the former at δ 1.43 ppm and the latter at 1.64 ppm).
Label the signals due to ha, hb, and hc in the 1h nmr spectra.
protons) give the same signal in the NMR whereas nonequivalent protons give different signals. For example, the compounds CH 3 CH 3 and BrCH 2 CH 2 Br all have one peak in their 1 H NMR spectra because all of the protons in each molecule are equivalent. The compound below, 1,2-dibromo-2-methylpropane, has two peaks: one at 1.87 ppm (the ... Browse by subjects in Label-the-signals-due-to-ha-hb-and-hc-in-the-1h-nmr-spectrum-of-acrylonitrile-ch2-5084407. No Subjects Found. About Business Homework Question and Answers. Crazy For Study is one of the leading providers of Business Textbook solution manuals for college and high school students. Get business textbook manual help and expert ... HA Degrees of # of unique signals in 0 3 unsaturation 13 C-NMR spectrum 1) Draw expanded structure and label ALL protons accordingly HA HB HC HD HE HF HG Integration CH2Cl2 Splitting (s,d,t,q…)
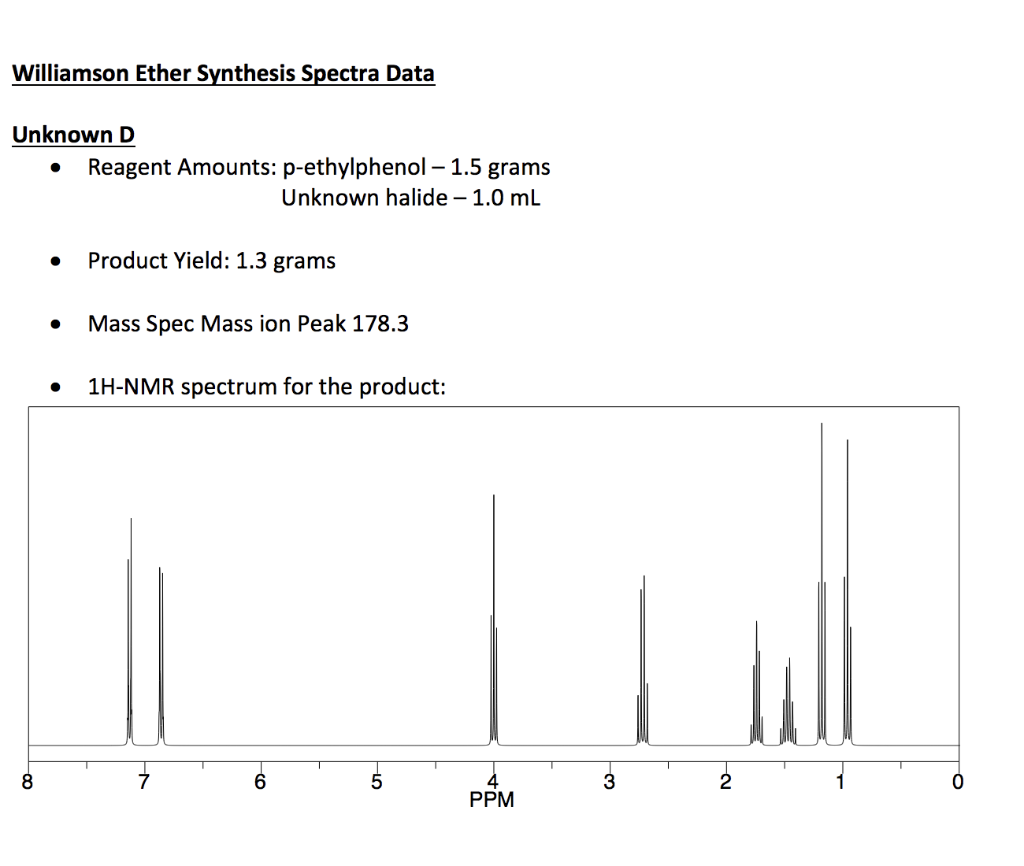
Label the signals due to ha, hb, and hc in the 1h nmr spectra.. Table 4 NMR data for C11H15NO2 number of ppm hydrogens multiplicity J (Hz) 1.30 3 triplet 7 3.00 6 singlet — 4.25 2 quartet 7 6.65 2 doublet 8 7.80 2 doublet 8 The two signals at 6.65 and 7.80 ppm, each of which integrates for two Chemical shift. The chemical shift is the position on the d scale (in ppm) where the peak occurs.; Typical d /ppm values for protons in different chemical environments are shown in the figure below. There are two major factors that influence chemical shifts (a) deshielding due to reduced electron density (due electronegative atoms) and (b) anisotropy (due to magnetic fields generated by π bonds). Label the signals due to Ha, Hb, and Hc in the 1 H NMR spectrum of acrylonitrile (CH2 CHCN). Draw a splitting diagram for the absorption due to the Ha ...1 answer · Top answer: CHEM Week 8 Notes • Solution – is a homogeneous mixture of 2 or more substances o Type solute = solution • Solutions have different electrolytic ... Assigning the 1H-NMR Signals of Aromatic Ring 1H-atoms Assigning 1H-NMR signals of 1H-atoms on an aromatic ring based upon their chemical shift and coupling can be accomplished in a number of different ways which will be detailed below. These methods which range from very simple to somewhat sophisticated are complimentary to one
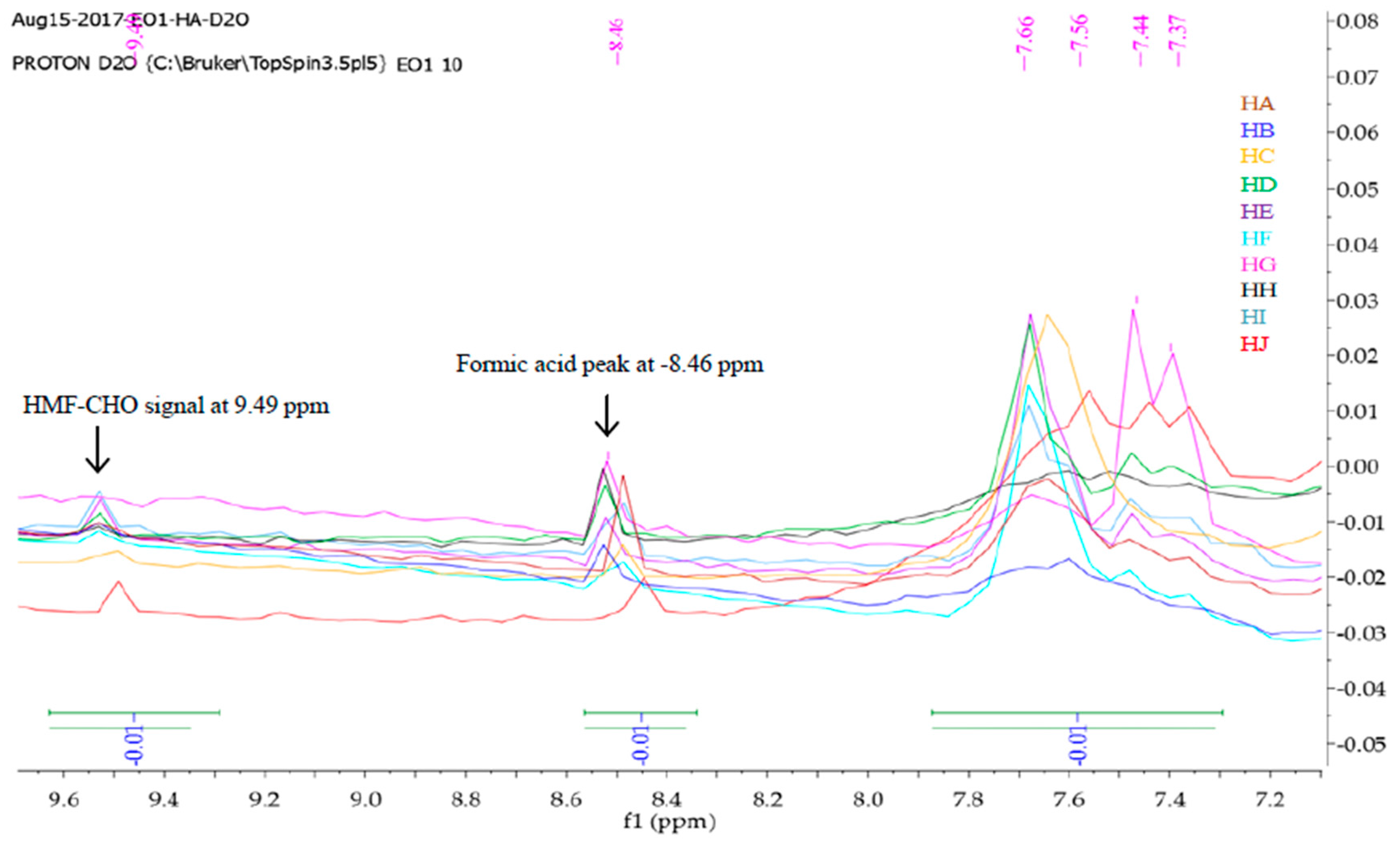
The 13 C-NMR spectra of Ini2 and Ini3 show the expected signals for the carbon atoms (e.g., a clear peak due to the C(H n )OH m group in Ini2 , which appears at 63.10 ppm, and a peak at 208.02 ppm, giving evidence for the existence of a keto Heller on today were doing Chapter fourteen from forty six on this farm. Give asked us to label the signal's due to Proton labeled a B and C and the strong molecule and coarse pawn them to where they fall in this anymore Spectra. And they also asked us to jaw splitting diagram for the absorption due to H April Tom. So let's first determine. So here we see that we have one, two, three ... Spectra. NMR. Exercises. Find the structure from 1H spectrum; 1H exercise generator; Assign 1H NMR spectra to molecule; 13C NMR; 1H NMR spectra of small molecules; 1H NMR spectra of Boc amino acids; Number of different Hs; 1H NMR integrate and find the structure; 1H number of signals; 1H NMR basic structure assignment; Tools. Multiplet ... 13C, 15N labeling + 2H labeling necessary!! 13C, 15N labeling. unfolded folded 15N 1H Is my sample OK for NMR? 1H-15N HSQC gives the protein fingerprint 15N 1H Signals of unfolded proteins have little1H dispersion, that means the 1H frequencies of all ... Ha, Hb (Leu50) N(Leu50) HN j Ha j
24 Apr 2018 · 1 answerStructure along with ¹H-NMR is shown below. Signal for Hₐ; Based on multiplicity of of the peak, a Singlet peak (the only singlet peak ... Ha Hb CH 3 Ha Br Hb 0.00 8.0 7.9 7.8 7.7 7.6 7.5 7.4 7.3 7.2 7.1 7.0 6.9 6.8 6.7 6.6 6.5 1.99 1.94 2.00 2.95 8 7 6 5 4 3 2 1 0 -1 39 13 C NMR Spectroscopy of Aromatic Compounds As with other 13C NMR spectra, aromatic compounds display single lines for each unique carbon environment in a benzene ring. 1. For each of the compounds shown below, determine the following: Number of signals in the 13C NMR spectrum, and · o Approximate chemical shift value for each signal Number of signals in the 'H NMR spectrum, and o o o Approximate chemical shift value for each signal Multiplicity (splitting) of each signal Relative integration of each signal (a) Worked example: WC NMR: 2 unique carbons are ... Splitting = n+1 , where n = the number of neighbouring protons For the protons Ha it doesn't have any n …. View the full answer. Transcribed image text: 9. Label the signals due to Ha, Hb, and Ho in the 1H NMR spectra. H O H H II H-C-C-C-C-H H. H.
Find the structure from 1H spectrum. 1H exercise generator. Assign 1H NMR spectra to molecule. 13C NMR. 1H NMR spectra of small molecules. 1H NMR spectra of Boc amino acids. Number of different Hs. 1H NMR integrate and find the structure. 1H number of signals.
Correct answer to the question Label the signals due to ha, hb, and hc in the 1h nmr spectra. - e-answersolutions.com
The 1H-NMR spectrum below is most likely of: Note: The proton NMR data (including the relative integration) are as follows: the broad singlet at 3.78 ppm (1H), the triplet at 3.67 ppm (2H), the triplet at 3.57 ppm (2H), and the pentet at 1.90 ppm (2H). l Cl OH ClC l CHO Cl OH O
9. (7 pts) a) Label the signals due to Ha, Hb and Hc in the 1H-NMR spectrum of acrylonitrile. b) Draw the splitting diagram for the absorption due to the Ha proton. 10. (6 pts) Explain how you could differentiate the following pairs of compounds by 1H-NMR. 11.
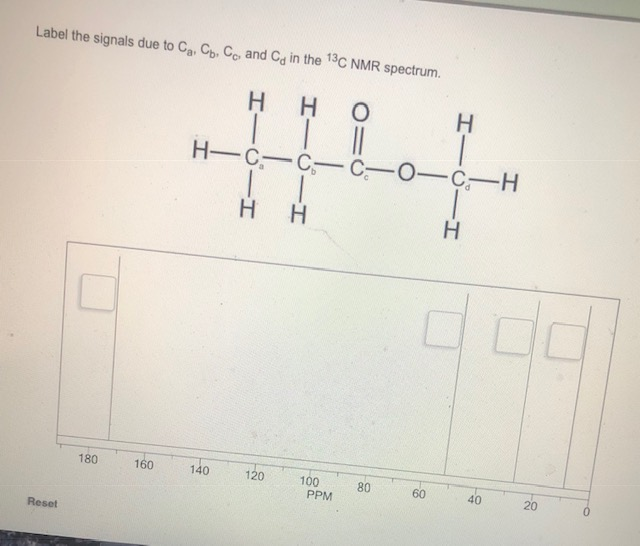
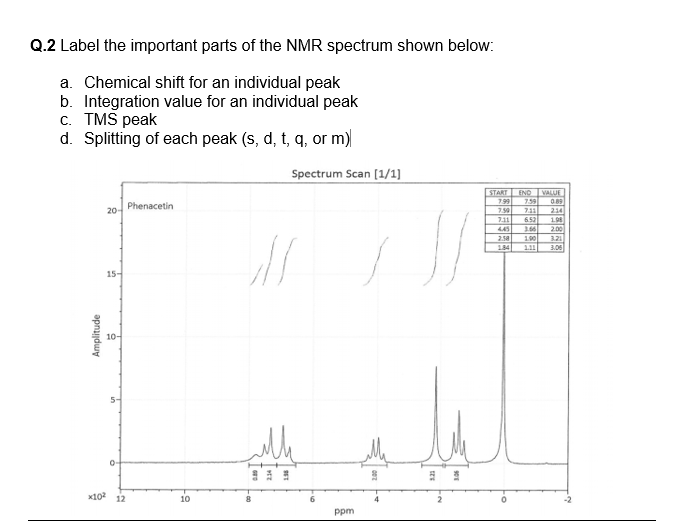
NMR spectroscopy is a great tool for determining structures of organic compounds. As you know 1H spectra have three features, chemical shift, signal intensity, and multiplicity, each providing helpful information. In this document we show how you use these features together to assign structures from 1H and 13C spectra. Use this approach.
Coupling in H-NMR. So far the H-NMR spectra that we have looked at have all had different types of protons that are seen as singlets in the spectra.This is not the normal case.... spectra usually have peaks that appear as groups of peaks due to coupling with neighbouring protons, for example, see the spectra of 1,1-dichloroethane shown below.
NMR of 4-methoxybenzaldehyde : NMR is a type of spectroscopy, which is used to determine the structure of a molecule. The signal split by following (n+1) ...1 answer · Top answer: As the signals split by the following (n+1) rule.so first find the number of equivalent hydrogens present in the adjacent carbon atom(s). Hence...
ANALYSIS OF 1H NMR SPECTRA INFORMATION CONTAINED IN PROTON NMR SPECTRA 1. NUMBER OF SIGNALS. The number of signal present in an NMR spectrum reflects the number of magnetically different protons. ... Ha Jab 15 Jac=10 Hb Jab 15 Jbc=2 Hc Jac=10 Jbc=2 In this example the splitting between protons a, b, and c does not follow the n+1 rule because ...
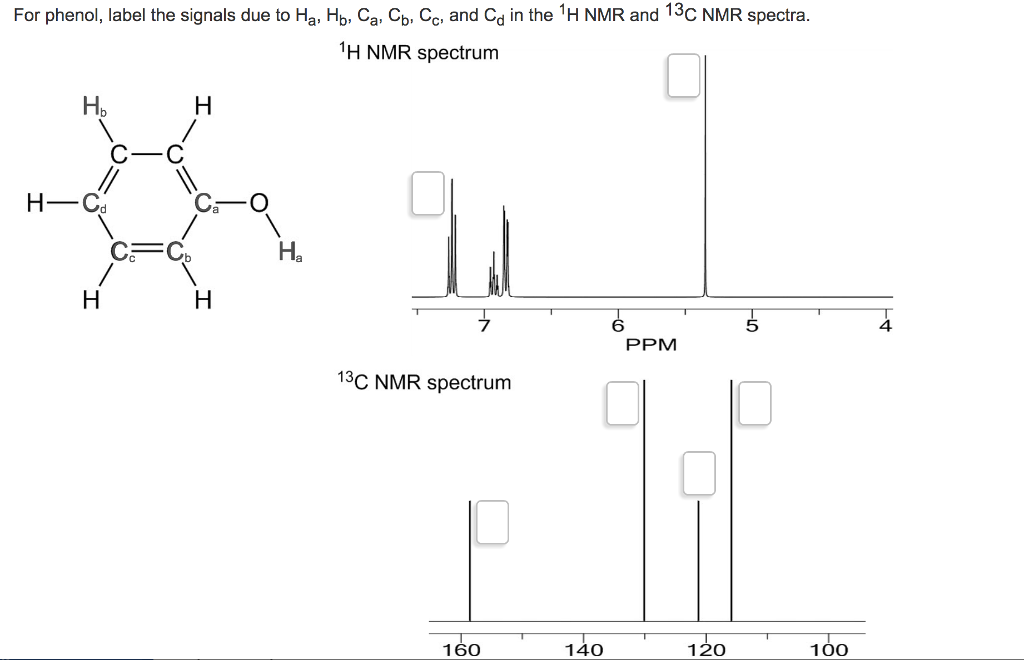
For phenol, label the signals due to Ha, Hb, ca, cb, cc, and cd in the 1H NMR and 13c NMR spectra. H NMR spectrum Hb C-C H- Cd C O C C Ha T T T T T T PPM 13 ...1 answer · 0 votes: Н Н Н— Са —Сь—Н Н Н Н Н a This is due to sp3 hybridized carbon which is sp2 carbon on alkene sp2 carbon This isdue to sp3 hybridized ...
CH14 Problem 46P Label the signals due to H a, H b, and H c in the 1 H NMR spectrum of acrylonitrile (CH 2 ==CHCN). Draw a splitting diagram for the absorption due to the H a proton. Step-by-step solution 100% (71 ratings) for this solution Step 1 of 4 Acrylonitrile has three set of protons, which are denoted as, and in the below diagram.
In the structure shown, Hb and Hc are classified as: Hb Ha A) homotopic protons. B) vicinal protons. C) enantiotopic protons. D) diastereotopic protons. E) isomeric protons. Ans: D Topic: Proton NMR- Chemical Shift, Splitting, Etc. 24. In the structure shown, Hb and Hc are classified as: HO H Ha Hb A) homotopic protons. B) vicinal protons.
aromatics, producing separate peaks. The NMR of bromobenzene is shown below. Notice the peaks that are shifted downfield (7.4-7.5 ppm). These H a couple to H b (J ortho = 6-10 Hz) which results in a doublet. The doublet is further split by coupling to H c with a very small J value (J para ~ 0-1 Hz). CH 3 Ha Ha Hb Hb Hc
The chemical shift range of 1H NMR is 0-14 ppm. In obtaining the NMR spectra for 1H NMR, continuous wave method is used. However, this is a slow process. Since the solvent does not contain any protons, 1H NMR spectra have no peaks for the solvent. What is 13C NMR. 13C NMR is used to determine the type and number of carbon atoms in a molecule.
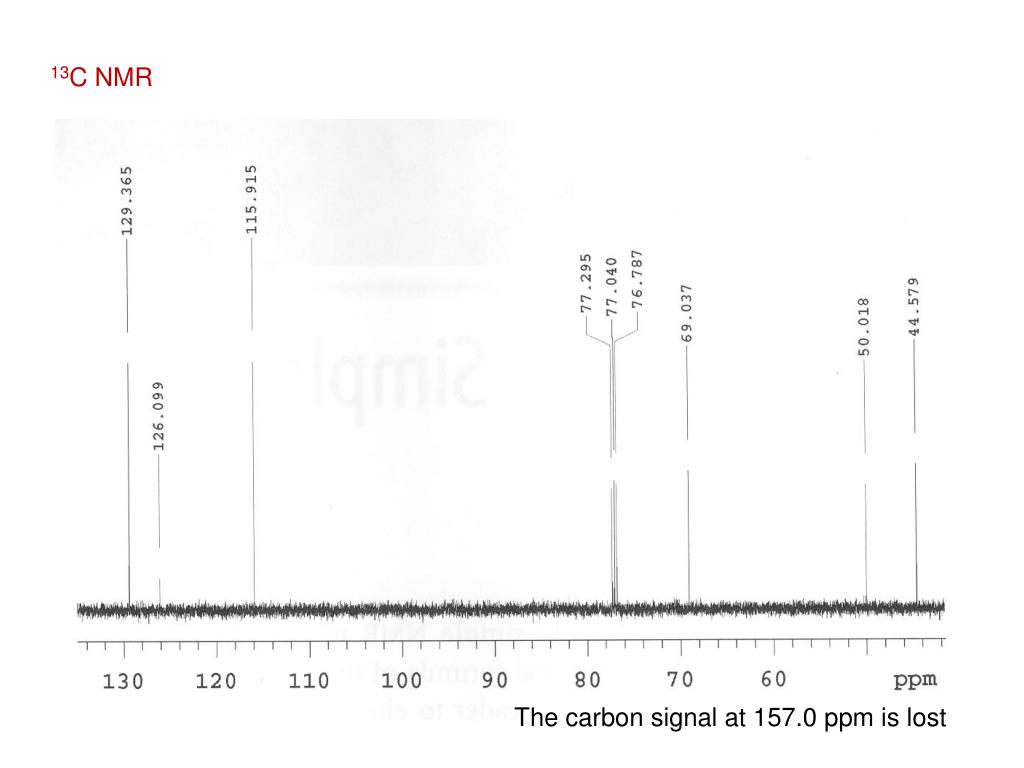
Nuclear Magnetic Resonance (NMR) Spectroscopy Dr. Vincent J. Storhaug Deuterated Solvent Signals in 13C NMR Spectra Why do you see this as a triplet at 77 ...
only given you partial spectra (from δ 3.0 - 7.0 ppm), showing the double bond regions. There are three peaks in this region in the carvone spectrum, corresponding to Ha, Hb and Hc. Based on chemical shift or coupling considerations, assign the peaks for Ha and Hb/c (you can't distinguish between Hb and Hc, so just label both peaks Hb/c).
How could the signals in the 6.5−8.1 -ppm region of their 1H NMR spectra distinguish among the following compounds?4 answers · Top answer: Hi guys. So for problem 59. We want to think about the signals that we would see between ...
H NMR Spectroscopy and Interpretation: More Detailed than the "Summary" 90 II. "Chemical Shifts" of the Signal Sets 9's (9.0-10.0) Aldehyde sp2 hybridized C-H's 7's (6.5-8.4) Aromatic sp2 hybridized C-H's 5's (4.8-6.8) Alkene sp2 hybridized C-H's 3's (2.8-4.5) Oxygenated sp3 hybridized C-H's (halogenated and nitrogenated alkyl C-H's will also come in this window ...
HA Degrees of # of unique signals in 0 3 unsaturation 13 C-NMR spectrum 1) Draw expanded structure and label ALL protons accordingly HA HB HC HD HE HF HG Integration CH2Cl2 Splitting (s,d,t,q…)
Browse by subjects in Label-the-signals-due-to-ha-hb-and-hc-in-the-1h-nmr-spectrum-of-acrylonitrile-ch2-5084407. No Subjects Found. About Business Homework Question and Answers. Crazy For Study is one of the leading providers of Business Textbook solution manuals for college and high school students. Get business textbook manual help and expert ...
protons) give the same signal in the NMR whereas nonequivalent protons give different signals. For example, the compounds CH 3 CH 3 and BrCH 2 CH 2 Br all have one peak in their 1 H NMR spectra because all of the protons in each molecule are equivalent. The compound below, 1,2-dibromo-2-methylpropane, has two peaks: one at 1.87 ppm (the ...

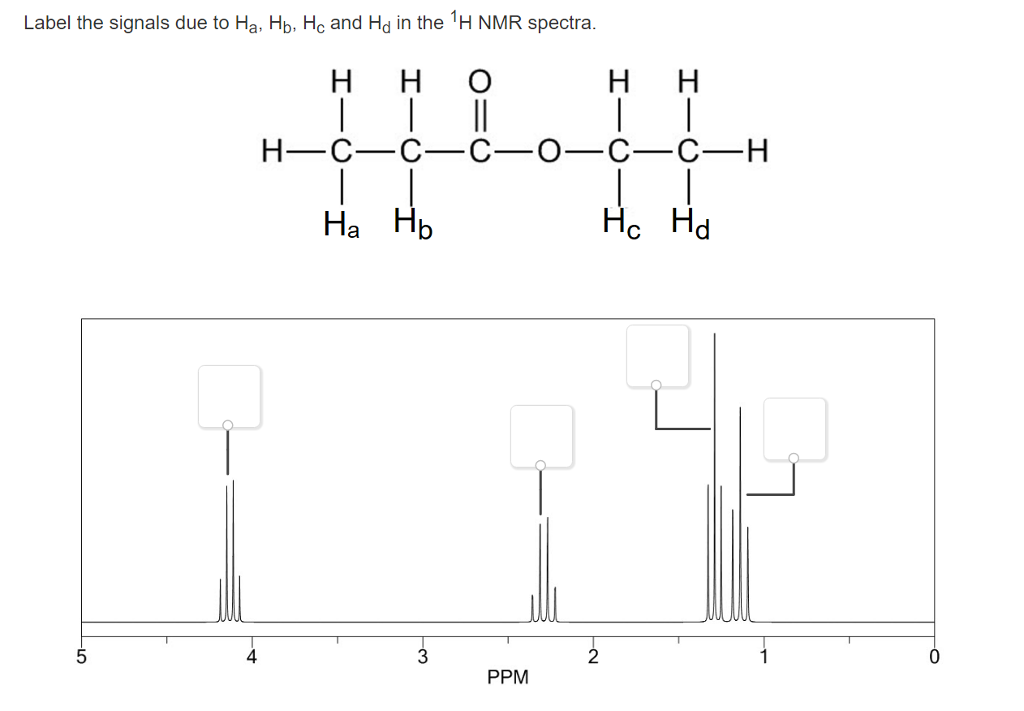



















0 Response to "38 label the signals due to ha, hb, and hc in the 1h nmr spectra."
Post a Comment