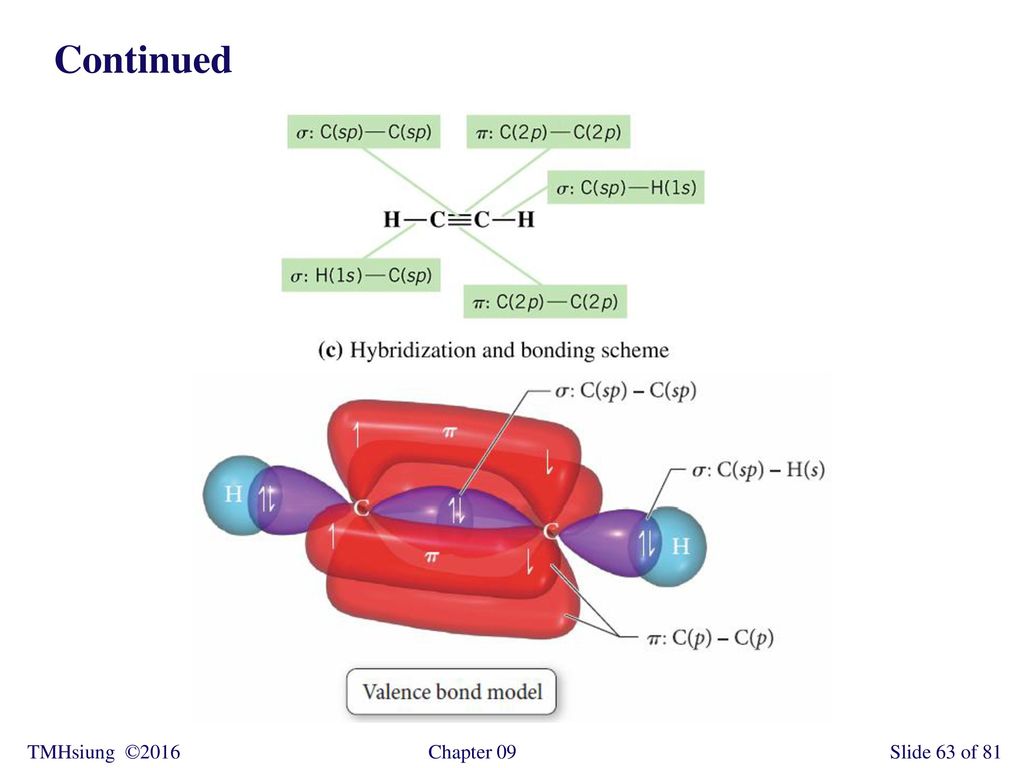
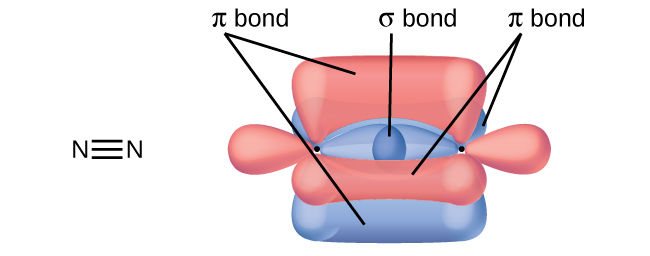
36 label all bonds using the σ and π notation followed by the type of overlapping orbitals.
35 Label All Bonds Using The σ And π Notation Followed By ... Label all bonds in hcn using the σ and π notation followed by the type of overlapping orbitals. Notation followed by the type of overlapping orbitals. Sketch the molecule beginning with the central atom and its orbitals showing overlap with the appropriate orbitals on the terminal atoms label all bonds using the σ or π notation followed by ... Overlapping of orbitals and its types, s-s, p-p, and s-p ... s - orbital is spherical in shape and overlapping takes place to some extent in all directions. Hence s -s bond is non - directional. Hydrogen (1s 1) atom has 1s orbital containing a single electron i.e. it is half-filled.Two such 1s orbitals from the two hydrogen atoms having electrons with opposite spins approach each other, then the potential energy of the system decreases.
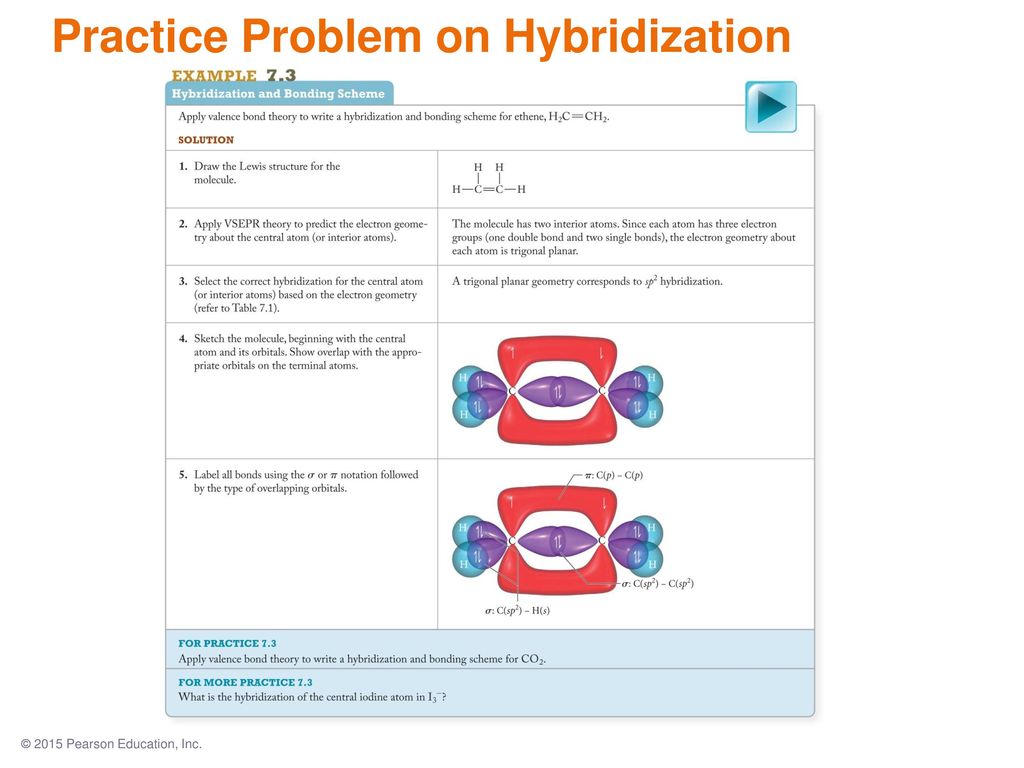
PDF Chemical Bonding II: Molecular Shapes, Valence Bond Theory ... Step 5 Label all bonds using the σ or π notation followed by the type of overlapping orbitals. Continued Example 10.6 Hybridization and Bonding Scheme
Label all bonds using the σ and π notation followed by the type of overlapping orbitals.
Chapter 8 Sample Problems (1).docx - Chapter 8 Sample ... Draw the hybridization scheme for the following molecules. Label all bonds using σ and π notation and by the type of overlapping orbitals. (a) N 2 H 4 (b) BrF 3 (c) SO 2 (d) XeF 4 Solved Label all bonds using the ? and ? notation followed ... In HCN, carbon is sp hybridized. Out of 3 p orbital, one (px) is involved in hybridization and 2 p orbitals (py and pz) remains unhybridized. The two sp hybrid orbitals form two sigma bonds with s orbital of hy …. View the full answer. Transcribed image text: Label all bonds the sigma and pi notation followed by the type of overlapping orbitals. Label the bonds in XeF4 using the σ sigmaa ... - Clutch Prep We're being asked to label the bonds in XeF 4 using the σ and π notation followed by the type of overlapping orbitals. Recall that σ (sigma) bonds are the single bonds in a molecule. They are formed from the end-on overlap of orbitals. For this problem, we need to do the following steps: Step 1: Determine the central atom in the molecule.
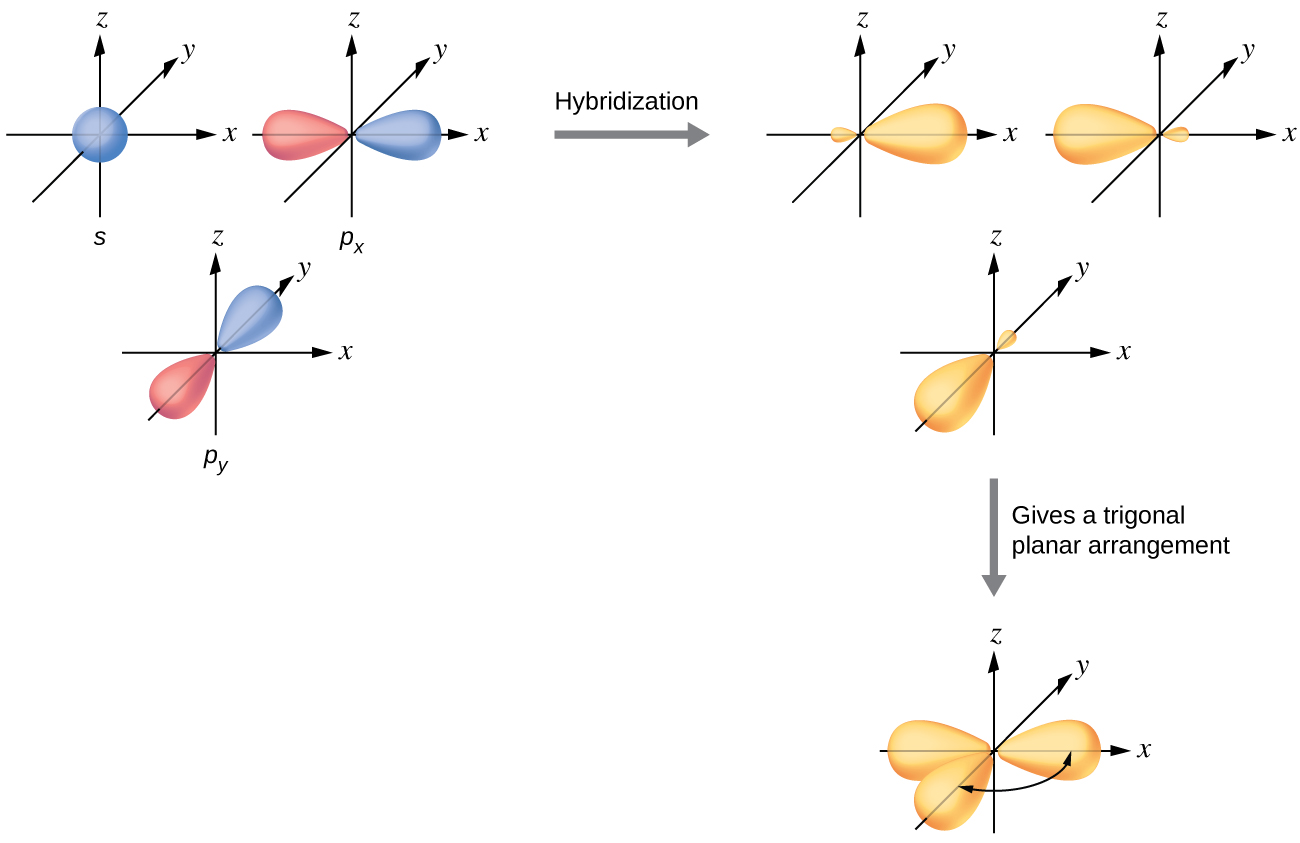
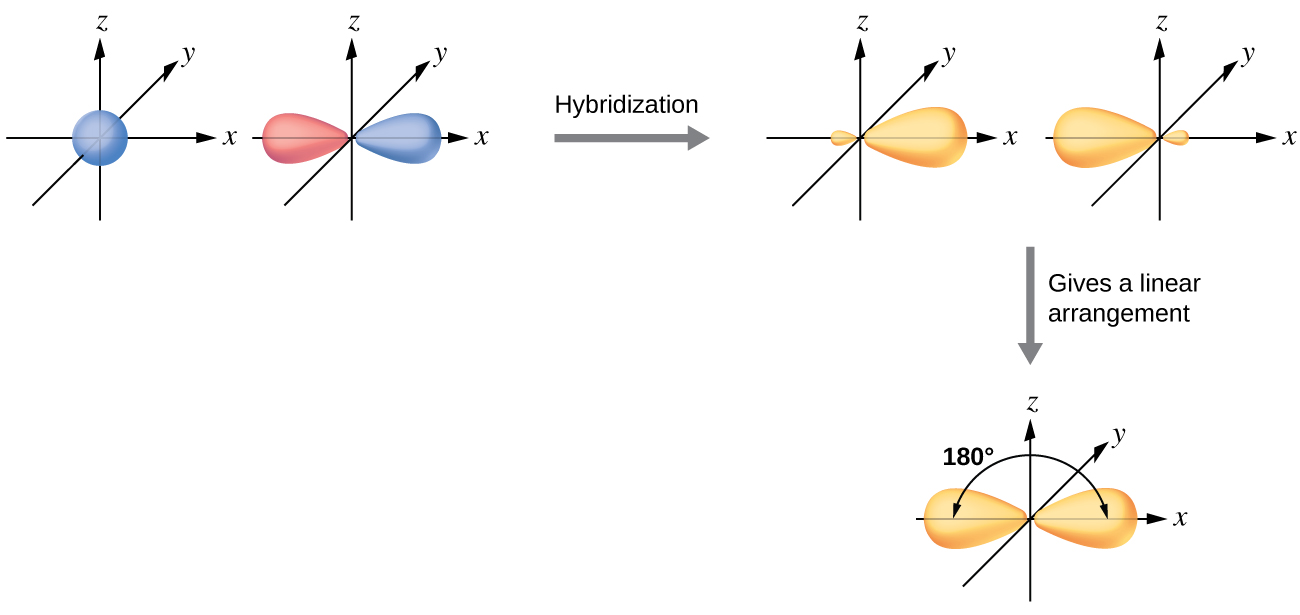
Label all bonds using the σ and π notation followed by the type of overlapping orbitals.. PDF Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals. PDF Orbital Picture of Bonding: Orbital Combinations ... ORBITAL PICTURE OF BONDING: ORBITAL COMBINATIONS, HYBRIDIZATION THEORY, & MOLECULAR ORBITALS ORBITAL COMBINATIONS Atomic orbitals can be combined and reshaped -much like dough- to make other orbitals of different shapes and properties. There are two basic types of orbitals that can result from such processes. They are: 1. HYBRID ORBITALS. Akdjhfadklhjkhdfhj A79 Nitrogen Trifluoride - helium gases he ballon grade, dipole moments ... akdjhfadklhjkhdfhj. akdjhfadklhjkhdfhj Chapter 10: Chemical Bonding II: Molecular Shapes, Valence ... Sketch the molecule, beginning with the central atom and its orbitals, showing overlap with the appropriate orbitals on the terminal atoms Label all bonds using the σ or π notation followed by the type of overlapping orbitals
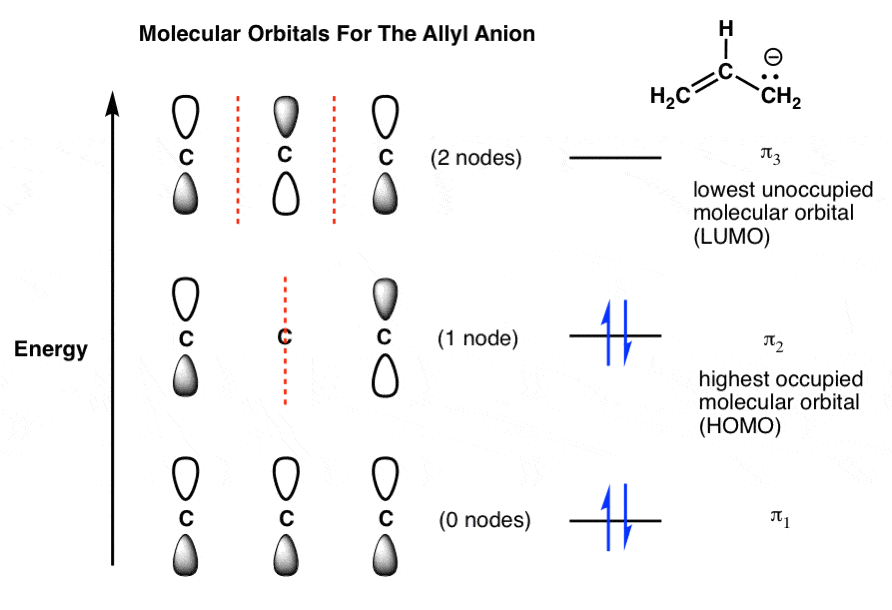
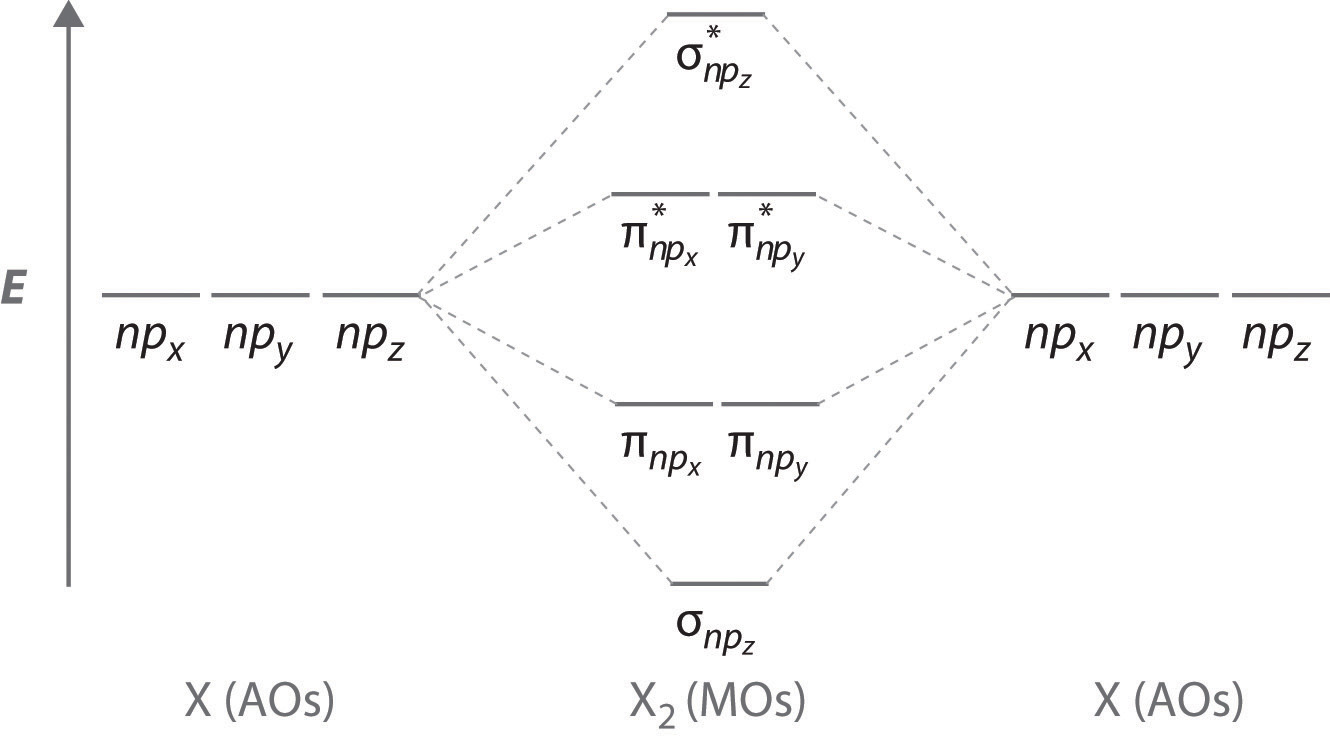
CHAPTER 10 CHEMICAL BONDING II Flashcards - Quizlet 5. label all bonds using sigma or pi bonds notation followed by the type of overlapping orbitals molecular orbital theory can approximate molecular orbitals as linear combination of atomic orbitals; total number of MOs formed from a particular set of AOs always equals the number of AOs in the set Sigma and Pi Bonds | Brilliant Math & Science Wiki Sigma and pi bonds are formed by the overlap of atomic orbitals. Sigma bonds are formed by end-to-end overlapping and Pi bonds are when the lobe of one atomic orbital overlaps another. Both acquired their names from the Greek letters and the bond when viewed down the bond axis. A sigma bond, 8.4 Molecular Orbital Theory - Chemistry Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals ( s, p, d …) and hybrid orbitals ( sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds. Label the bonds between xenon and fluorine using the σ and ... σ: Xe( Sp3d2) - F(p) Explanation: Step one: write the ground state configuration and the excited state configuration of the xenon atom. Ground state= [Kr] 5s2. 4d10. 5p6. The Sp3d2 hybrid will be the impaired electrons will bond with the fluorine atom. For pi, π bonding we have; Xe(Sp3d2) - F(p), Xe(sp3d) - F(s), Xe(sp3d) - F(p) and Xe(Sp3d2) - F(s).
30 Label All Bonds Using The σ And π Notation Followed By ... Label all bonds using the. Label all bonds using the σ and π notation followed by the type of overlapping orbitals.. Drag the appropriate labels to their respective targets. Label the bonds between xenon and fluorine using the and notation followed by the type of overlapping orbitals. Expert answer 100. Valence Bond Theory (Vbt) | Hybridization | Sp | Sp2 | Sp3 ... Depending on the types of orbitals overlapping, the σ-bond is divided into following types: σ s-s bond: σ p-p bond: σ s-p bond: (ii) π-bond: The covalent bond formed by sidewise overlapping of atomic orbitals is called π- bond. In this bond, the electron density is present above and below the inter nuclear axis. Sigma and Pi Bonds - Definition and Detailed Explanation The electrons participating in a σ bond are commonly referred to as σ electrons. Generally, all single bonds are sigma bonds. They can be formed via the following combinations of atomic orbitals. S-S Overlapping. In this kind of overlapping, one 's' orbital from each participating atom undergoes head-on overlapping along the internuclear ... Solved For XeF4 Label the bonds between xenon and ... - Chegg Label the bonds between xenon and fluorine using the σ and π notation followed by the type of overlapping orbitals. Label the bonds between xenon and fluorine using the and notation followed by the type of overlapping orbitals. σ:Xe(sp3d2)−F(p) σ:Xe(sp3d)−F(p) π:Xe(sp3d)−F(p) σ:Xe(sp3d2)−F(s) π:Xe(sp3d2)−F(p) σ:Xe(sp3d)−F(s)
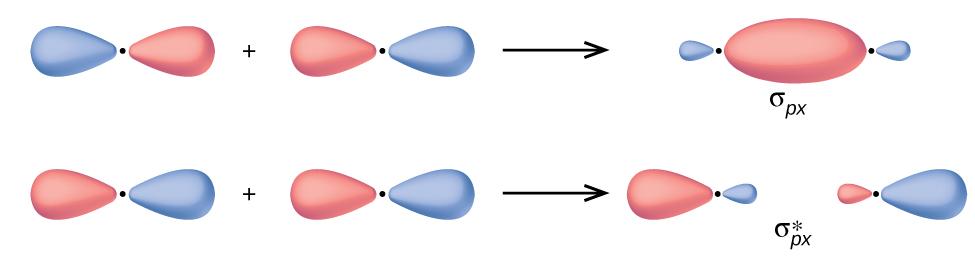
PDF ORBITALS and MOLECULAR REPRESENTATION OVERLAPPING ORBITALS Chemical bonds are formed from the overlapping of atomic orbitals having the same phase. Overlapping orbitals of opposite phase form antibond- ing orbitals. BONDING ORBITALS ANTIBONDING ORBITALS s + s σ s - s σ* s + p σ s - p σ* p + p σ p - p σ* p + p π p - p π* ORBITALS AND MOLECULAR REPRESENTATION 9 ENERGY 1sA1sB HAH2HB
+Fluorineb.) orbital Notation - Brainly.ph Fluorine b.) orbital Notation 1 See answer
Answered: Sketch XeF2 showing the orbitals and… | bartleby Sketch XeF2 showing the orbitals and any overlapping orbitals to indicate covalent bonds. Also, label all bonds using sigma or pi notation followed by the type of overlapping orbitals. Sketch XeF 2 showing the orbitals and any overlapping orbitals to indicate covalent bonds.
Label the bonds between xenon and fluorine using the σ and ... Label the bonds between xenon and fluorine using the σ and π notation followed by the type of overlapping orbitals. - Let's ques! Posted on. October 26, 2021 by hakan.
Step 3 Determine the geometry that minimizes the repulsion ... View CHEM2210_Chapter 10 Packet.pdf from CHE 1210 at RMU. CHEM2210 Dr. T Chapter 10 Packet The VSEPR Model (Valence-shell electron repulsion): predicts the shapes of molecules and ions by assuming
Label all bonds in HCN using the sigma σ a... | Clutch Prep Q. Label the bonds in XeF4 using the σ sigmaand π pinotation followed by the type of overlapping orbitals. Q. The structure of acetylsalicylic acid (aspirin) is shown below.How many pi bonds are present in acetylsalicylic acid?
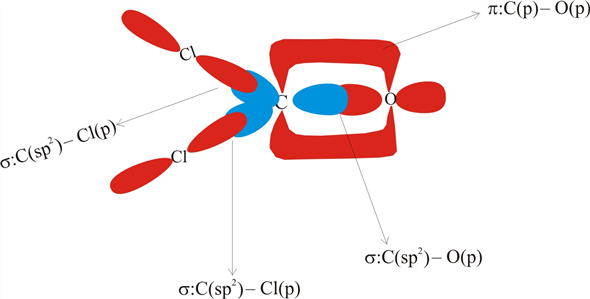
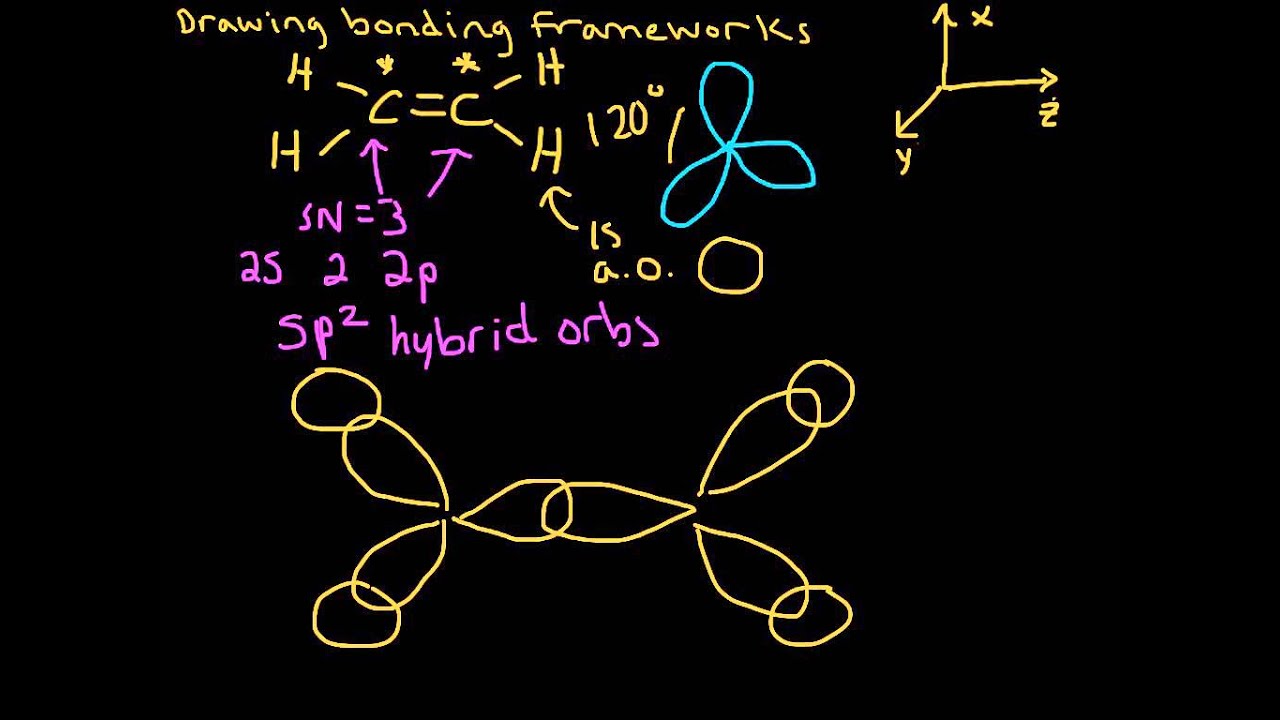
PDF Chapter 14 Covalent Bonding: Orbitals The two C‒Cl σ bonds are formed from overlap of sp2 hybrids from C with sp3 hybrid orbitals from Cl. The double bond between the carbon and oxygen atoms consists of one σ and one π bond. The σ bond in the double bond is formed from head-to-head overlap of an sp2 orbital from carbon with an sp2 hybrid orbital from oxygen.
how many σ bonds are in acetylsalicylic acid No menu assigned! how many σ bonds are in acetylsalicylic acid. By February 15, 2021 Uncategorized
31 Label All Bonds In So2. - Labels For Your Ideas Solved Label All Bonds In Ch2br2 Label All Bonds In So2 If they cancel out the molecule is nonpolar. Label all bonds in so2.. Label all bonds using the σ or π notation followed by the type of overlapping orbitals. By analyzing the lewis structure of so2 we can see that the so2 is asymmetrical because it contains a region with different sharing.
Label the bonds in XeF4 using the σ sigmaa ... - Clutch Prep We're being asked to label the bonds in XeF 4 using the σ and π notation followed by the type of overlapping orbitals. Recall that σ (sigma) bonds are the single bonds in a molecule. They are formed from the end-on overlap of orbitals. For this problem, we need to do the following steps: Step 1: Determine the central atom in the molecule.
Solved Label all bonds using the ? and ? notation followed ... In HCN, carbon is sp hybridized. Out of 3 p orbital, one (px) is involved in hybridization and 2 p orbitals (py and pz) remains unhybridized. The two sp hybrid orbitals form two sigma bonds with s orbital of hy …. View the full answer. Transcribed image text: Label all bonds the sigma and pi notation followed by the type of overlapping orbitals.
Chapter 8 Sample Problems (1).docx - Chapter 8 Sample ... Draw the hybridization scheme for the following molecules. Label all bonds using σ and π notation and by the type of overlapping orbitals. (a) N 2 H 4 (b) BrF 3 (c) SO 2 (d) XeF 4












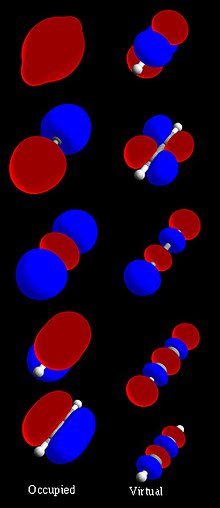

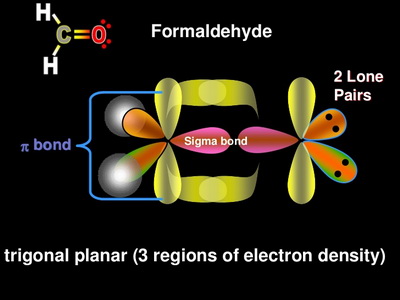



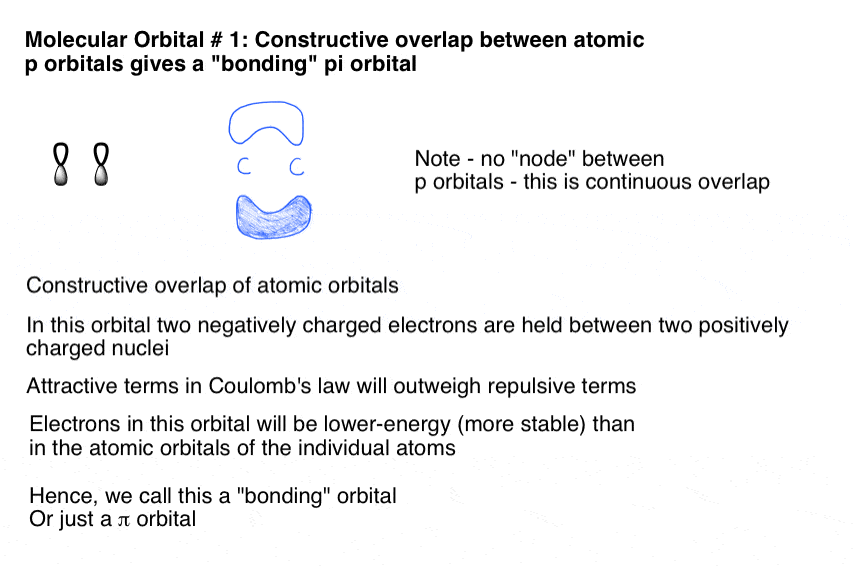

0 Response to "36 label all bonds using the σ and π notation followed by the type of overlapping orbitals."
Post a Comment