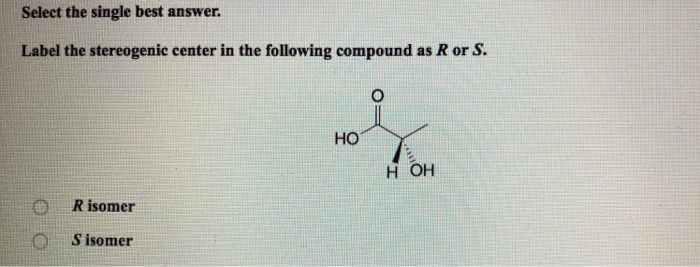
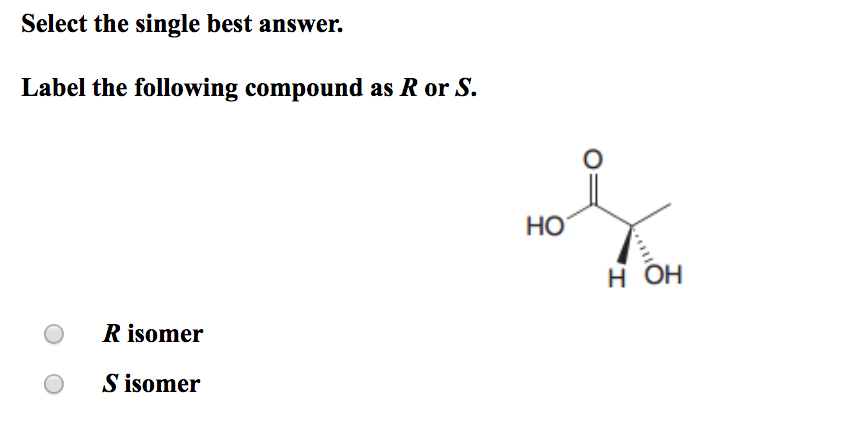
36 label the following compound as r or s.
SOLVED:Label each stereogenic center as R or S. You must be signed in to discuss. In chemistry, an organic c…. In chemistry, the structur…. Label each stereogenic cen…. Assign R, S designations …. Label each compound as R …. Assign R or S configur…. Assign R or S configur…. Assign R or S configur…. R/S - Two Stereogenic Centers The R/S Naming System. Two or more Stereogenic Centers. Optical Activity. R/S Naming. Diastereoisomerism. Meso Compounds. Today, we'll look at naming compounds with stereocenters, and then we'll examine the complications which arise when a molecule has more than one stereocenter in it. First, though, let's look at a property in which one ...
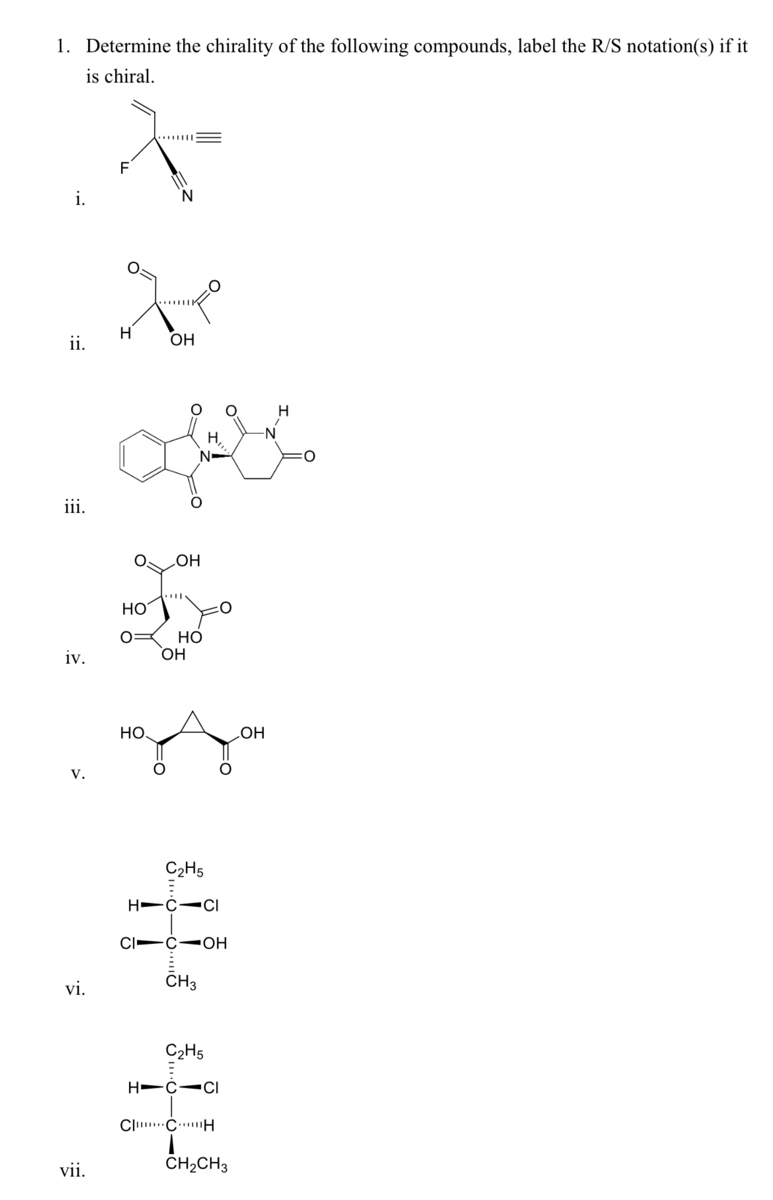
The R and S Configuration Practice Problems - Chemistry Steps In the previous post, we talked about the Cahn-Ingold-Prelog rules for assigning the R and S configuration. In the following practice problems, we will assign the R and S configuration for bond-line representations and use it to determine the relationship between the two compounds (identical, enantiomers or diastereomers), and also assign the R and S configuration on Fischer projections:
Label the following compound as r or s.
Finding R and S for Chiral Centers - Organic Chemistry ... It is a stereochemical label to indicate the relative spatial orientation of each atom in a molecule with a non-superimposable mirror image. R indicates that a clockwise circular arrow that goes from higher priority to lower priority crosses over the lowest priority substituent and that lowest-priority substituent is in the back. PDF Chirality Practice Problems (Answer Key) - SI PROGRAM 3. Determine the R or S configuration for each of these compounds. Note that they are all Fischer Projections. 4. Classify each of the following pairs of molecules as enantiomers, diastereomers, or identical. Label any molecules that are meso. Keep in mind the "most powerful definitions" for each of these stereoisomers. Explain, please... S or R? Label the following compound as ... Label the following compound as R or S. R or S Configuration The (R) or (S) configuration is assigned by the Cahn-Ingold-Prelog convention in fisher projections. The groups which are connected to...
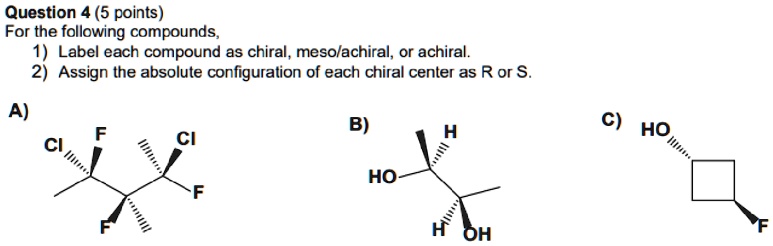
Label the following compound as r or s.. Solved 2) Label the following compounds as having R or S ... 2) Label the following compounds as having R or S configuration around the stereocenter (s).2) Label the following compounds as having R or S configuration around the stereocenter (s). Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Stereochemistry Worksheet 213-1.pdf - CHM 345 Lab ... Label (by circling or marking with an asterisk) all the stereo-centers on the following molecules. Note: you can't label their absolute configurations (R or S) because there is no wedge/dash scheme. 4. Label the following compounds as having an R or S configuration around their stereocenter(s). SOLVED:Draw both enantiomers of each amino acid and label ... Video Transcript. This is the answer to Chapter 19. Problem number 21 from the Smith Organic Chemistry textbook. Ah, and this problem says, draw both an anti murders of each amino acid and label them as our or s on the two amino acids were asked to draw our fennel Alan in and a thiamine. Organic Chemistry I_Quiz 5 (1).pdf - Organic Chemistry I ... (2 pts) 5. A. Locate the stereogenic centers in the following compound. B. What is the maximum number of stereoisomers possible for the following compound? (3 pts) 6. a) Label each stereogenic center as R or S in the following compound.
PDF Organic Chemistry I CHEM 2323 Packet #3 1. Consider the ... 1. Consider the following molecule. Label all chiral atoms as either R or S. Draw the compound in Newman projection (looking down the C2-C3 bond) and Fischer projection. Draw a diastereomer in Newman projection. Need to change one of the chiral centers for a diastereomer. If both chiral centers are changed would obtain the enantiomer. Chapter 5 TB Flashcards - Quizlet 52) Label each asymmetric carbon in the compound below as R or S. 54) Draw the structure of the enantiomer of (2S, 3R)-2,3-dichloropentane. Take particular care to indicate three-dimensional stereochemical detail properly. [Solved] Please see an attachment for details | Course Hero 1. there are 3 chiral centers, where each carbon has 4 substituents that are different. 2. using the formula 2 n = 2 3 = 8 stereoisomers. 3. The molecule is shown with wedges and dashes with their perspective R or S configuration, in this case it is drawn as configurations all containing R stereochemistry Chiral Centers Questions-Answers - Q- Label the following ... View Test Prep - Chiral Centers Questions-Answers from CH 328M at University of Texas. Q- Label the following compounds R or S if they are chiral. If there are not chiral write A in the box for
PDF Chapter 4: Stereochemistry Worksheet 1. Draw the following ... 1. Draw the following compounds and label the Chiral carbon(s). 3 -chloropentane 3-chloro-2-methylpentane 2-bromo- I -chlorobutane 2. Define the Cahn, Ingold, Prelog Sequence Rules. 3. List the three rules for assigning the R/S prefixes. 4. Draw the Fischer projection of I ,2-Dichlorobutane. 5. Draw the Fischer projection of (S)- I -Chloro-2 ... PDF Practice Questions for Ch. 5 Part I 8) Label each asymmetric carbon in the compound below as R or S. OH CH3 9) Label each asymmetric carbon in the compound below as R or S. OH H CH3 OH H CH3 10) Label each assymetric carbon in the compound below as R or S. Cl 11) Draw the structure of (2R,3S)-2,3-dichloropentane. Take particular care to indicate three-dimensional stereochemical ... PDF Chemistry 2500 (Fall 2017): Assignment #9 Stereochemistry 5. Where appropriate, label all stereogenic centers as R or S and all double bonds as E or Z. No explanation is required. a) H 2 N H 3 C C H 2 C H 2 CH 2 Br b) CH 2 Cl C CH 3 CH 2 O H O H c) P H 3 C C(CH 3) 3 H 6. Draw all possible stereoisomers of this compound and indicate which are enantiomers and which are diastereomers. Label all ... How to Determine the R and S configuration - Chemistry Steps Carbon is not the only atom designated by R and S. In theory, any atom with four different groups is chiral and can be described by the R and S system. For example, phosphorous and sulfur chiral centers are often assigned as R or S. Hydrogen is not always the lowest priority. A lone pair of electrons is lower.
Honors English 12 A Unit 4: The Renaissance: 1485 - Quizlet Terms in this set (218) Aesop, who lived from 620 to 560 BC, was a writer of fables. complex sentence. We can infer from Aristotle's descriptions that Aesop was a freed slave, but little else is known about him. compound-complex sentence. Some scholars doubt that he ever lived. complex sentence.
Label the hydrophilic and hydrophobic parts in the ... Label the hydrophilic and hydrophobic parts in the following compounds. (i) C H 3 (C H 2 ) 1 0 C H 2 O S O 3 N + a (ii) C H 3 (C H 2 ) 1 5 N + (C H 3 ) 3 B r (iii) C H 3 (C H 2 ) 1 6 C O O (C H 2 C H 2 O) n C H 2 C H 2 O H
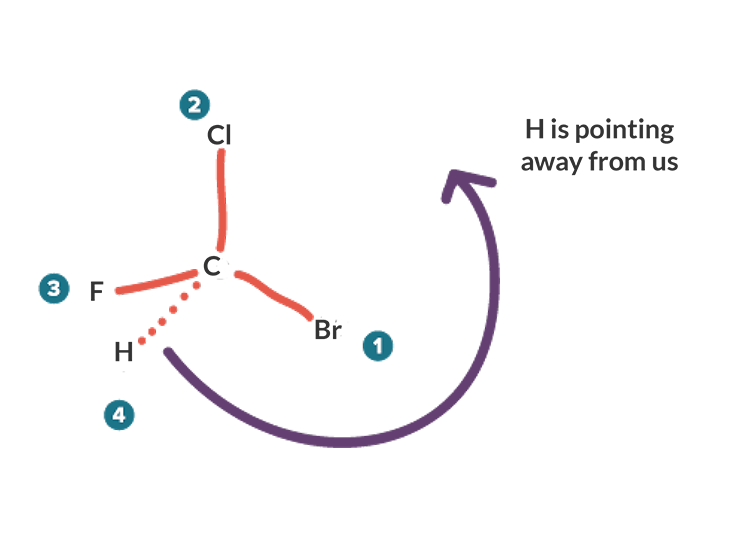
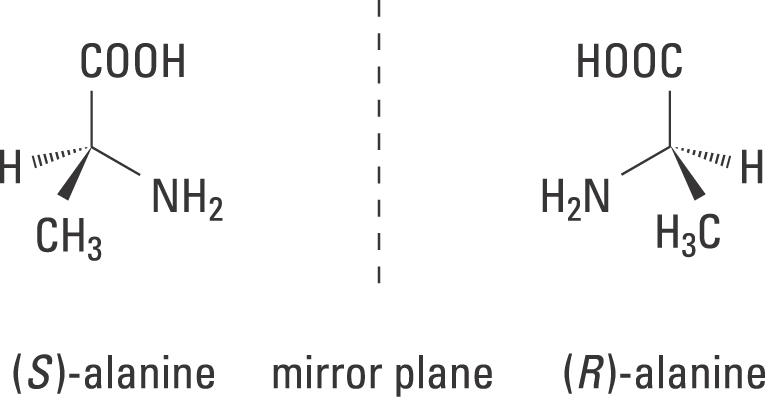
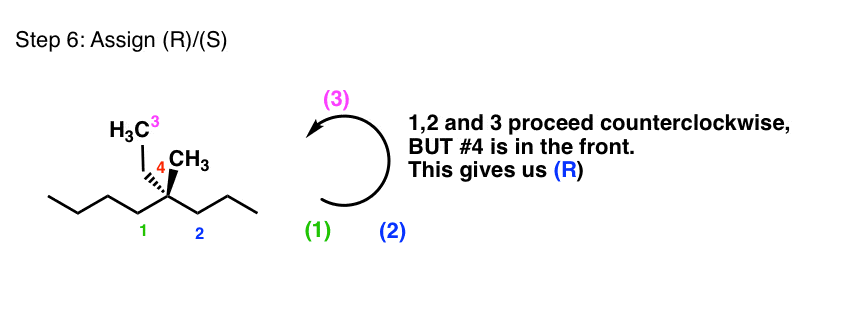
5.6: Labeling Stereogenic Centers with R or S - Chemistry ... Stereocenters are labeled R or S. The "right hand" and "left hand" nomenclature is used to name the enantiomers of a chiral compound. The stereocenters are labeled as R or S. Consider the first picture: a curved arrow is drawn from the highest priority ( 1) substituent to the lowest priority (4) substituent.
Solved Label the following compounds as having R or S ... Question: Label the following compounds as having R or S configuration around the stereocenter(s). This problem has been solved! See the answer See the answer See the answer done loading
Answered: Q1/ label each sterogenic centern in… | bartleby Q1/ label each sterogenic centern in the following compounds as (R) or (S)- OH но 4. utit H. Question. Transcribed Image Text: Q1/ label each sterogenic centern in the following compounds as (R) or (S). OH HC но OH OH 4a 7a Expert Solution. Want to see the full answer? Check out a sample Q&A here.
ORGANIC CHEMISTRY 089.docx - Draw and label the ... Draw and label the enantiomers of each of the following compounds as either R or S: (a) 4-chloro-2-pentene (c) isopentylbenzene (b) isobutyl alcohol (d) phenylalanine Solution: (a) 4-chloro-2-pentene Since there is one chiral center (C*),two (2 1) enantiomeric forms exist for this compound: According to the rules of sequence, the order of priority for the four groups is: H, the atom of the ...
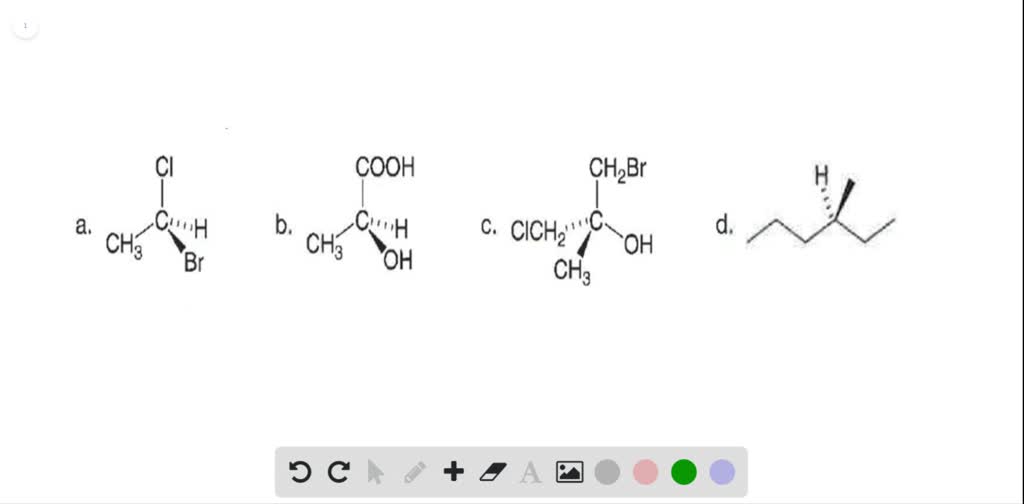
PDF v= (R) CI LR) - csus.edu Identify the following pairs of compounds as enantiomers, diastereomers, constitutional isomers, or the same. a. diastereomers b. enantiomers 15. Identify the stereocenters in the following molecules and indicate whether they are R or S. 16. Using the tests for chirality we have learned, determine whether the following molecules are chiral.
5.3 Chirality and R/S Naming System - Organic Chemistry I For following compounds, label each of the chirality center with a star. Approach: The carbons in CH 3 or CH 2 are NEVER chirality centers. The chirality center must be the carbon bonded with a branch (or branches). sp 2 double bond carbon is NEVER a chirality center.
What the **** OChem???? Flashcards - Quizlet Label each asymmetric carbon in the compound below as R or S. One S, Two R Phantasmidine, shown below, is found in poisonous frog skin and has analgesic properties (J. Nat. Prod. 2010, 331).
Explain, please... S or R? Label the following compound as ... Label the following compound as R or S. R or S Configuration The (R) or (S) configuration is assigned by the Cahn-Ingold-Prelog convention in fisher projections. The groups which are connected to...
PDF Chirality Practice Problems (Answer Key) - SI PROGRAM 3. Determine the R or S configuration for each of these compounds. Note that they are all Fischer Projections. 4. Classify each of the following pairs of molecules as enantiomers, diastereomers, or identical. Label any molecules that are meso. Keep in mind the "most powerful definitions" for each of these stereoisomers.
Finding R and S for Chiral Centers - Organic Chemistry ... It is a stereochemical label to indicate the relative spatial orientation of each atom in a molecule with a non-superimposable mirror image. R indicates that a clockwise circular arrow that goes from higher priority to lower priority crosses over the lowest priority substituent and that lowest-priority substituent is in the back.


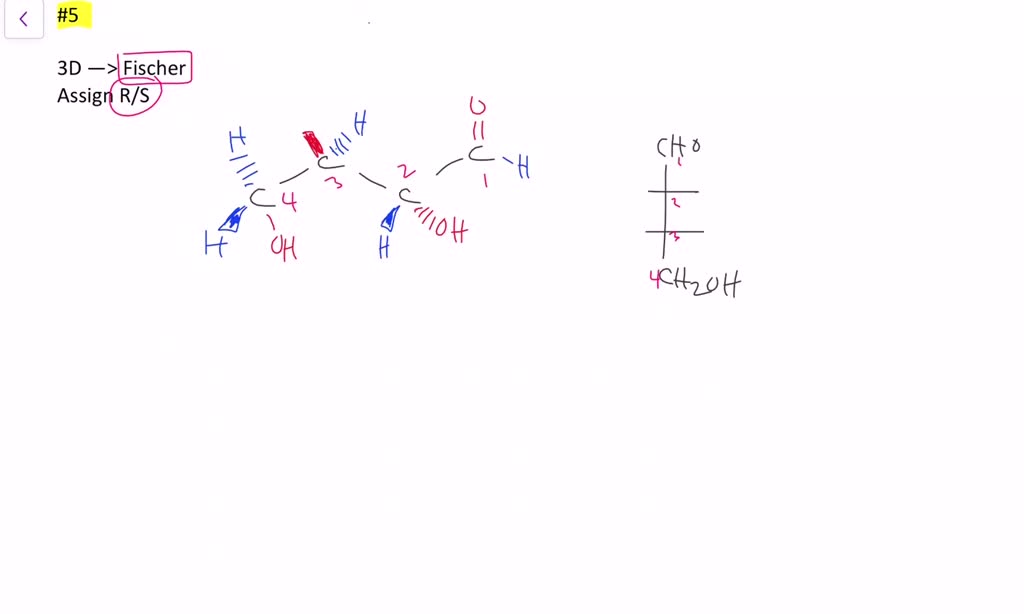
















0 Response to "36 label the following compound as r or s."
Post a Comment