37 viibryd off label uses
How Long Does it Take for Viibryd Work? - Addiction Group Viibryd is approved by the FDA to treat major depressive disorder (MDD). It has other "off-label" uses, too. How Fast Does Viibryd Work? Sleep, energy, or appetite usually improve within the first 1 to 2 weeks. These are important indications of how well the medicine will work long-term. Viibryd: Uses, Dosage & Side Effects Information - Drugs.com Viibryd ( vilazodone) is an antidepressant in a group of drugs called selective serotonin reuptake inhibitors (SSRIs). Viibryd is used to treat major depressive disorder (MDD). It is not known if Viibryd is safe and effective for use in children for the treatment of MDD. Warnings
Mental Health Medications | NAMI: National Alliance on ... Vilazodone (Viibryd) expand. Vortioxetine (Trintellix) expand. Ziprasidone (Geodon) expand. Selecting Medications expand. Medication Plan Adherence expand. What to Avoid with Psychiatric Medications expand. What to Expect From Your Medications expand. Considerations for Special Groups expand. Off-Label Usage of Medications expand. Generic ...

Viibryd off label uses
PDF Reference ID: 2894777 - Food and Drug Administration VIIBRYD. is not approved for use in pediatric patients [see Warnings and Precautions (5.1), Use in Specific Populations (8.4), and Patient Counseling Information (17.1)] 1 INDICATIONS AND USAGE . VIIBRYD is indicated for the treatment of major depressive disorder (MDD). The efficacy of VIIBRYD was established in two 8-week, randomized, double-blind, PDF Clinical Policy: Vilazodone (Viibryd) 1. Refer to the off-label use policy for the relevant line of business if diagnosis is NOT specifically listed under section III (Diagnoses/Indications for which coverage is NOT authorized): HIM.PA.154 for health insurance marketplace and CP.PMN.53 for Medicaid. II. Continued Therapy A. Depression (must meet all): 1. EOF
Viibryd off label uses. Viibryd Medication: How to Treat Drug Abuse Some of this drug's off-label uses are for the treatment of anxiety disorders, bipolar disorders, chronic pain, vasomotor disorders, and post-traumatic stress disorder. Viibryd For Depression. Viibryd medication falls into the drug class of antidepressants - specifically, SSRI. Dosing Options | VIIBRYD® (vilazodone HCl) Gradual dose reduction rather than abrupt cessation is recommended whenever possible. Viibryd should be tapered from the 40 mg once daily dose to 20 mg once daily for 4 days, followed by 10 mg once daily for 3 days. Patients taking Viibryd 20 mg once daily should be tapered to 10 mg once daily for 7 days. Viibryd (vilazodone): Side effects, dosage, interactions ... It is used off-label for generalized anxiety disorder and is being studied for other off-label conditions. Mechanism of action. The exact mechanism of action of Viibryd is unknown. Viibryd: Uses, Side Effects and Dosage - Verywell Mind Off-Label Uses Even though it isn't approved by the FDA specifically for the treatment of anxiety disorders, some research shows that Viibryd is effective in treating generalized anxiety disorder. So, in some cases, doctors may prescribe Viibryd and an off-label treatment for anxiety. 2 7 Types of Depression You May Not Know About Before Taking
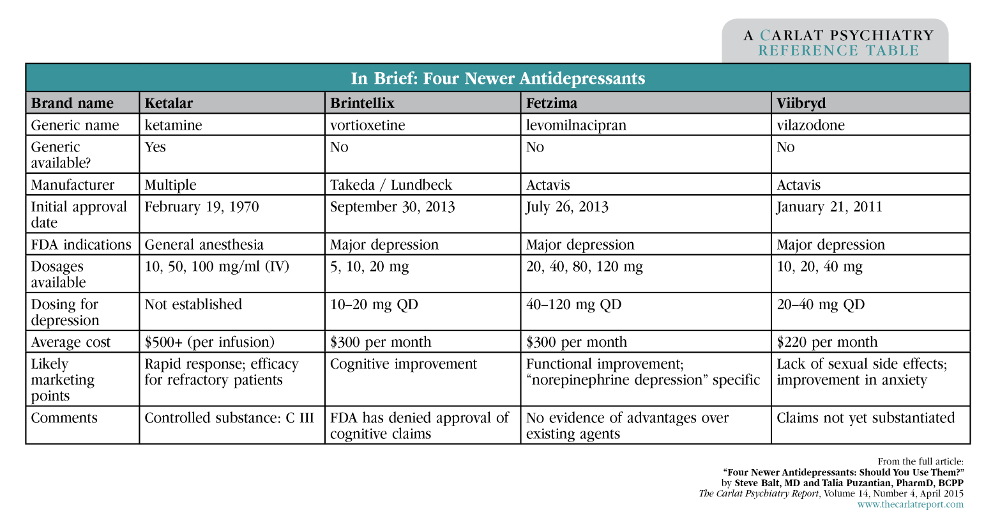
Remeron Weight Gain: How To Manage Body Mass on Mirtazapine 25.11.2019 · Mirtazapine, which is generic Remeron, belongs to the drug class called tetracyclic antidepressants (TeCAs) and its main use is for the treatment of major depressive disorder. In 2019, around 1.2 million patients in the United States used Mirtazapine, and weight gain, as one of its side effects, is an object of many studies at the moment.. This drug can cause mass build … DOC Pharmacy Benefits Management Services Home Potential off-label uses: Other antidepressants have label indications for or are used off-label to treat anxiety disorders, bipolar depression, PTSD, chronic pain, and vasomotor symptoms. Results of a search of PubMed and clinical trials.gov found vilazodone has been studied only as a treatment for MDD. PDF Full Prescribing Information Viibryd is not approved for use in pediatric patients (8.4). RECENT MAJOR CHANGES . Warnings and Precautions (5.9) 7/2021 . INDICATIONS AND USAGE . VIIBRYD is indicated for the treatment of major depressive disorder (MDD) in adults (1). DOSAGE AND ADMINISTRATION Recommended target dosage: 20 mg to 40 mg once daily with food (2.1, 12.3). Four Newer Antidepressants: Should You Use Them? Carlat ... Since 2011, 3 new antidepressants have been approved by the FDA, and another (ketamine) has been generating buzz as a potential off-label medication for depression. In this article, we'll take a step back and review the data on vilazodone (Viibryd), levomilnacipran (Fetzima), vortioxetine (Brintellix), and ketamine. Vilazodone (Viibryd)
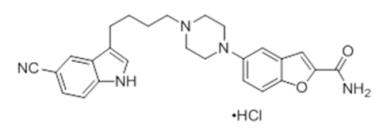
Four Newer Antidepressants: Should You Use Them? Since 2011, 3 new antidepressants have been approved by the FDA, and another (ketamine) has been generating buzz as a potential off-label medication for depression. In this article, well take a... Psychopharmacopeia: Online Searchable Psychopharmacology ... vilazodone VIIBRYD. vilazodone. VIIBRYD. Class: SPARI / Phenylpiperazine-2-benzofurancarboxamide. FDA Indications: MDD. Off-Label Use: Anxiety. PDF Clinical Policy: Vilazodone (Viibryd) Vilazodone (Viibryd ®) is an antidepressant. FDA Approved Indication(s) Viibryd is indicated for the treatment of major depressive disorder. Policy/Criteria Provider must submit documentation (such as office chart notes, lab results or other clinical information) supporting that member has met all approval criteria. Viibryd - FDA prescribing information, side effects and uses Viibryd is a prescription medicine used to treat a certain type of depression called Major Depressive Disorder (MDD) in adults. It is not known if Viibryd is safe and effective for use in children for the treatment of MDD. Who should not take Viibryd? Do not take Viibryd if you: take a Monoamine Oxidase Inhibitor (MAOI)
Major Depressive Disorder Treatment | VIIBRYD® (vilazodone ... VIIBRYD and other antidepressants may increase suicidal thoughts or actions in some people 24 years of age and younger, especially within the first few months of treatment or when the dose is changed. VIIBRYD is not for use in children. Depression or other serious mental illnesses are the most important causes of suicidal thoughts or actions.
Trintellix: Side effects, dosage, uses, and more Trintellix is not FDA-approved for use by children. However, it may be used off-label for children. In one clinical study of children ages 7 to 17 years, the most common side effects included:...
EOF
PDF Clinical Policy: Vilazodone (Viibryd) 1. Refer to the off-label use policy for the relevant line of business if diagnosis is NOT specifically listed under section III (Diagnoses/Indications for which coverage is NOT authorized): HIM.PA.154 for health insurance marketplace and CP.PMN.53 for Medicaid. II. Continued Therapy A. Depression (must meet all): 1.
PDF Reference ID: 2894777 - Food and Drug Administration VIIBRYD. is not approved for use in pediatric patients [see Warnings and Precautions (5.1), Use in Specific Populations (8.4), and Patient Counseling Information (17.1)] 1 INDICATIONS AND USAGE . VIIBRYD is indicated for the treatment of major depressive disorder (MDD). The efficacy of VIIBRYD was established in two 8-week, randomized, double-blind,
























:max_bytes(150000):strip_icc()/GettyImages-1223673234-0f4e05d60d884f1d90c630e66d60c192.jpg)

0 Response to "37 viibryd off label uses"
Post a Comment