38 label the anode and cathode. indicate the direction of electron flow.
Answered: 1.) Draw a voltaic cell for the ... - bartleby Label the anode and cathode, indicate the direction of electron flow, and identify the contents of each half cell and the direction of ion flow through the salt bridge. Write the correct half-cell notation for this reaction below your drawing. 2.) (Get Answer) - a Sketch the galvanic cell described. Label ... a Sketch the galvanic cell described. Label the positive and negative electrodes. Mark the direction of the electron flow. b Write the half-equations for the reactions that occur in each half-cell and an equation for the overall reaction. c Label the anode and cathode. d Indicate the direction in which ions in the salt bridge migrate.
Determine the direction of electron flow and label the ... Correct answers: 3 question: Determine the direction of electron flow and label the anode and the cathode. Label each electrode as negative or positive. Indicate the direction of anion and cation flow in the salt bridge
Label the anode and cathode. indicate the direction of electron flow.
OneClass: Draw an electrolytic cell in which Mn2+ is ... Part A:Label the anode and cathode, indicate the direction of electron flow. Part B: Write an equation for the half-reaction occurring at each electrode. Express your answers as chemical equations separated by a comma. Identify all of the phases in your answer. Solution: Sketch a voltaic cell for each r ... - Clutch Prep Label the anode and cathode and indicate the half-reaction that occurs at each electrode and the species present in each solution. Also indicate the direction of electron flow. b. 2 H + (aq) + Fe (s) → H 2 (g) + Fe 2+ (aq) Learn this topic by watching Galvanic Cell Concept Videos Frequently Asked Questions Solution: Make a sketch of a concentratio... | Chemistry The concentration of Zn2+ in one of the half-cells is 2.0 M and the concentration in the other half-cell is 1.0 X 10-3 M. Label the anode and the cathode and indicate the half-reaction occurring at each electrode . Also indicate the direction of electron flow.
Label the anode and cathode. indicate the direction of electron flow.. Chapter 20 Problems.pptx - CHAPTER 20 ... - Course Hero By using data from Appendix E, determine E° red for the reaction involving Pd. c. Sketch the voltaic cell, label the anode and cathode, and indicate the direction of electron flow. Problem 20.54: If the equilibrium constant for a one-electron redox reaction at 298 K is 8.7 × 10 4 , calculate the corresponding ΔG° and E°. (Get Answer) - Draw out a cell for Al in Al(NO 3 ) 3 and ... Label the anode and cathode sides, including charge on each electrode. (hint: use a table of standard reduction potentials). Indicate direction of electron flow. Write the net reaction, and the half reactions. What is the standard cell potential? Indicate for both electrodes whether they are dissolving, growing, or staying the same size. Answered: Sketch a voltaic cell for each redox… | bartleby Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that occurs at each electrode and the species present in each solution. Also indicate the direction of electron flow. a. 2 Ag*(aq) + Pb(s) → 2 Ag(s) + Pb²+(aq) b. Label the anode and cathode, indicate the ... | Clutch Prep FREE Expert Solution We're being asked to label the anode and cathode, indicate the direction of electron flow and the species present in each solution for the reaction. Ni2+(aq) + Mg (s) → Ni (s) + Mg2+(aq) We'll identify the anode and cathode half-reactions using the following steps. Step 1. Separate the overall reaction into two half-reactions
Chem 180 Exam 3 Flashcards - Quizlet Label the anode and cathode, identify the sign of each electrode, and indicate the direction of electron and ion flow. 2Al(s)+3Cd2+(aq)→2Al3+(aq)+3Cd(s) Write the anode half-reaction. Draw a diagram for this Galvanic cell, labeling the ... Positive or negative terminal Electrons always flow from the negative terminal to the positive terminal- exactly the opposite as the direction of the current flow. Therefore the cathode would be the positive terminal and the anode the negative terminal. References [1] " Galvanic Cells ", CK-12 Editor, a. Label the anode and cathode. - Numerade Label the anode and cathode. b. Indicate the direction of electron flow. c. Indicate what happens to the concentration of Pb2+ in each half-cell. Answer. a) In concentration cells, the two half cells have same electrodes and electrolytes but the concentrations of the solutions are different. PPTX Electrochemistry - Oneonta Label the anode and the cathode, showing the corresponding half-reactions. Indicate the direction of electron flow in the external circuit. SO42- Al Al3+ + 3e- e- e- anode Cu2+ + 2e- Cu cathode Cu2+ Cu Al3+ NO3- Al CA2+ NO3- 2 Voltaic cell notationis a shorthand method of describing a voltaic cell.
Answered: Hello, 86) Make a sketch of an ... - Bartleby.com Science Chemistry Q&A Library Hello, 86) Make a sketch of an electrochemical cell with the overall reaction shown here. Label the anode, the cathode, and the salt bridge. Indicate the direction of electron flow. Hint: When drawing electrochemical cells, the anode is usually drawn on the left side. Mg (s) + Ni2+ (aq) -----> Mg2+ (aq)+ Ni (s) Thanks, Determine the direction of electron flow and label the ... Apr 26, 2018 · Determine the direction of electron flow and label the anode and the cathode. label each electrode as negative or positive. write a balanced equation for the overall reaction 2 See answers Advertisement Answer 5.0 /5 6 JABARBS At anode is where oxidation takes place, that is Cr (s)→Cr3+ (aq) +3e- this is the oxidation Draw an electrolytic cell in which Mn2+ is reduced to... Problem: Draw an electrolytic cell in which Mn2+ is reduced to Mn and Sn is oxidized to Sn2+.Label the anode and cathode, indicate the direction of electron flow, and write an equation for the half-reaction occurring at each electrode. Solved Label the anode and cathode and indicate the ... Label the anode and cathode and indicate the direction of electron flow for the following overall redox reaction: 2C102(g) + 21- (aq) +2010, (aq) + 12(s) Drag the appropriate labels to their respective targets. Reset Help | Cathode Anode C10, I G1 G2 G2 C1O2 G3 G3 Submit Request Answer Part F Indicate the half-reaction occurring at the cathode.
Show the direction of electron flow and ion flow Label the ... Show the direction of electron flow and ion flow. Label the anode and cathode and their sign. The direction of electron flow is from a) Cu to Ag b) Ag to Cu The direction of ion flow is from a) the Cu 2+ to Ag + solution b) the Ag + to Cu 2+ solution Does your cell look like your neighbors cell? a) Yes b) No
Answered: Draw an electrolytic cell in which Mn2+… | bartleby Label the anode and cathode, indicate the direction of electron flow, and write an equation for the half-reaction occurring at each electrode. What minimum voltage is necessary to drive the reaction? Draw an electrolytic cell in which Mn2+ is reduced to Mn and Sn is oxidized to Sn2+.
How do you sketch galvanic cells? + Example - Socratic.org You draw the anode, cathode, the oxidizing and reducing agents, a porous plate or a salt bridge. You show the direction of electron flow in the external circuit and the flow of ions in the internal circuit. EXAMPLE: Sketch a cell diagram for the reaction Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s) Solution: A. Draw one of the diagrams above (no labels).
Chapter 17, Electrochemistry Video Solutions ... - Numerade (b) Label the anode and cathode. (c) Indicate the direction of electron flow in the wire and ion flow in the solutions. (d) Tell what electrolyte could be used in the salt bridge, and indicate the direction of ion flow. (e) Write balanced equations for the electrode and overall cell reactions.
Solved Label the anode and cathode and indicate the ... Transcribed image text: Label the anode and cathode and indicate the direction of electron flow for the following overall redox reaction 02(g) + 4H+ (aq) +2Zn(s) → 2H2O(1) +2Z0?* (aq) Drag the appropriate labels to their respective targets. Indicate the half-reaction occurring at the anode. Express your answer as a chemical equation. Identify all of the phases in your answer.
(c) Indicate the direction of electron flow in ... - Numerade (a) Label the electrodes, and identify the ions present in the solutions. (b) Label the anode and cathode. (c) Indicate the direction of electron flow in the wire and ion flow in the solutions. (d) Tell what electrolyte could be used in the salt bridge, and indicate the direction of ion flow.
18 24 The following picture of a galvanic (b) Label the anode and cathode. (c) Indicate the direction of electron flow in the wire and ion flow in the solutions. Tell what electrolyte could be used in the salt bridge, and indicate the direction (d) of ion flow. Write balanced equations for the electrode and overall cell reactions. (e)
Solved Part A Label the anode and cathode. Indicate the ... Chemistry questions and answers. Part A Label the anode and cathode. Indicate the direction of electron flow. Drag the appropriate labels to their respective targets Cathode Anode Pb () Pb (s) Salt bridge containing 2.5 M Pb2 5.0x 10M Pb2 Reset Help Submit My Answers Give Up. Question: Part A Label the anode and cathode.
Draw an electrolytic cell in which Mn2+ is reduced to Mn ... Answers: 1 on a question: Draw an electrolytic cell in which Mn2+ is reduced to Mn and Sn is oxidized to Sn2+. (Assume standard conditions). Part A Label the anode and cathode, indicate the direction of electron flow. Drag the appropriate labels to their respective targets. Part B Write an equation for the half-reaction occurring at each electrode. Express your answers as chemical equations ...
Draw an electrolytic cell in which Mn2+ is ... - Brainly.com Electrons flow from anode to cathode as indicated. The battery connected to the set up drives this non spontaneous electrolytic process. Oxidation half equation; Sn (s) ------> Sn^2+ (aq) + 2e Reduction half equation: Mn^2+ (aq) + 2e ----> Mn (s) Cell voltage= E°cathode - E°anode E°cathode= -1.19V E°anode= -0.14 V Cell voltage= -1.19 V - (-0.14V)
Solution: Make a sketch of a concentratio... | Chemistry The concentration of Zn2+ in one of the half-cells is 2.0 M and the concentration in the other half-cell is 1.0 X 10-3 M. Label the anode and the cathode and indicate the half-reaction occurring at each electrode . Also indicate the direction of electron flow.
Solution: Sketch a voltaic cell for each r ... - Clutch Prep Label the anode and cathode and indicate the half-reaction that occurs at each electrode and the species present in each solution. Also indicate the direction of electron flow. b. 2 H + (aq) + Fe (s) → H 2 (g) + Fe 2+ (aq) Learn this topic by watching Galvanic Cell Concept Videos Frequently Asked Questions
OneClass: Draw an electrolytic cell in which Mn2+ is ... Part A:Label the anode and cathode, indicate the direction of electron flow. Part B: Write an equation for the half-reaction occurring at each electrode. Express your answers as chemical equations separated by a comma. Identify all of the phases in your answer.
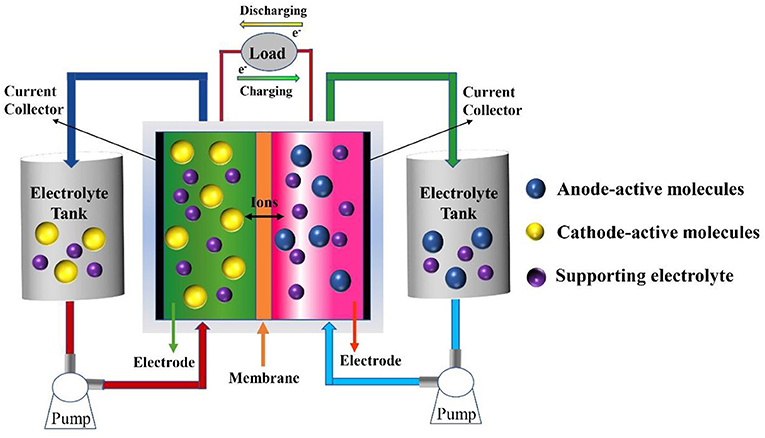


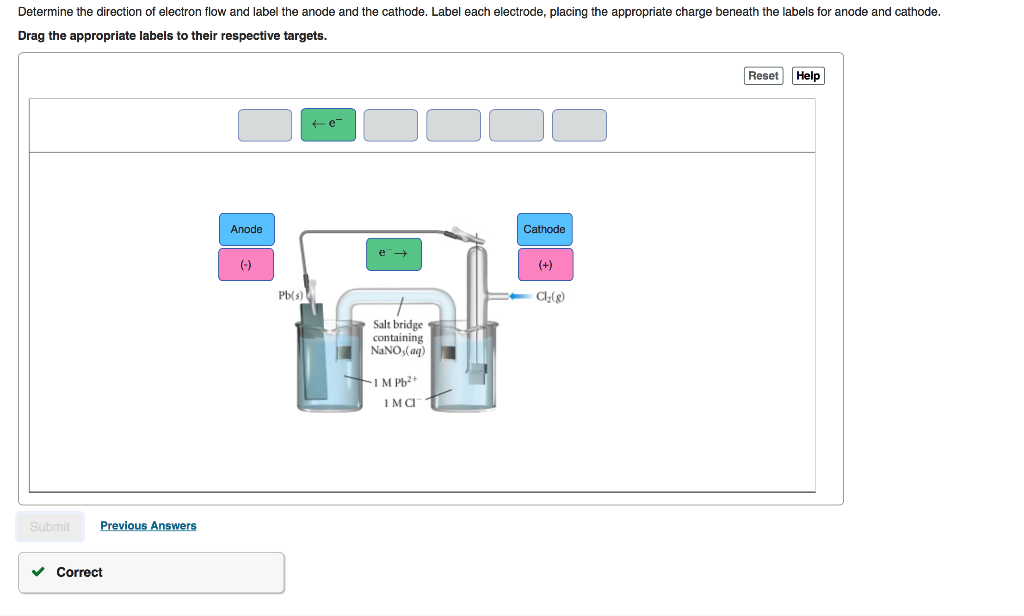
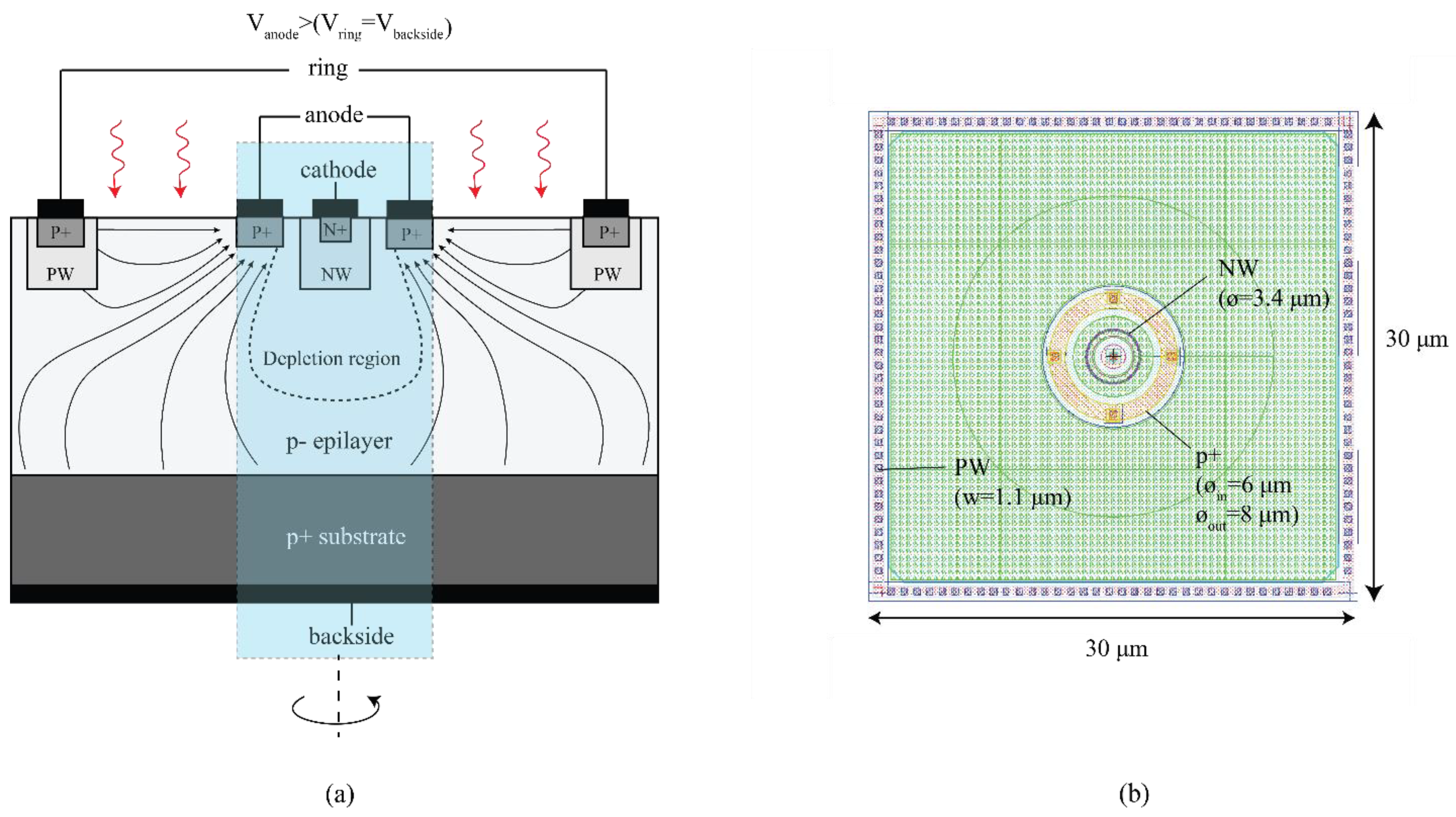
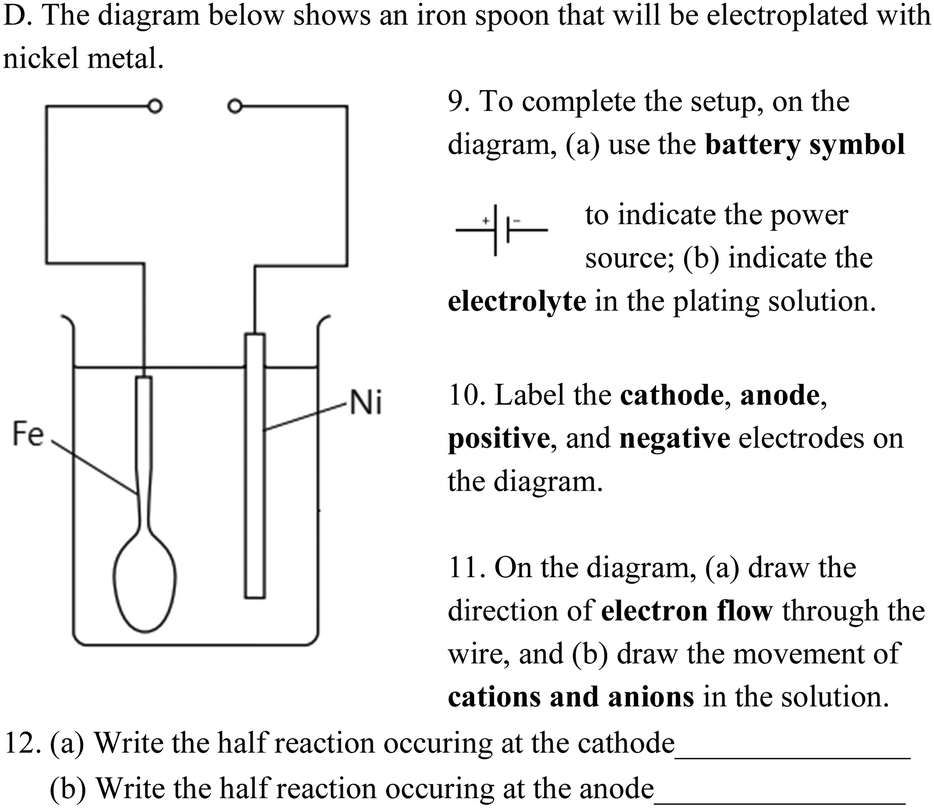




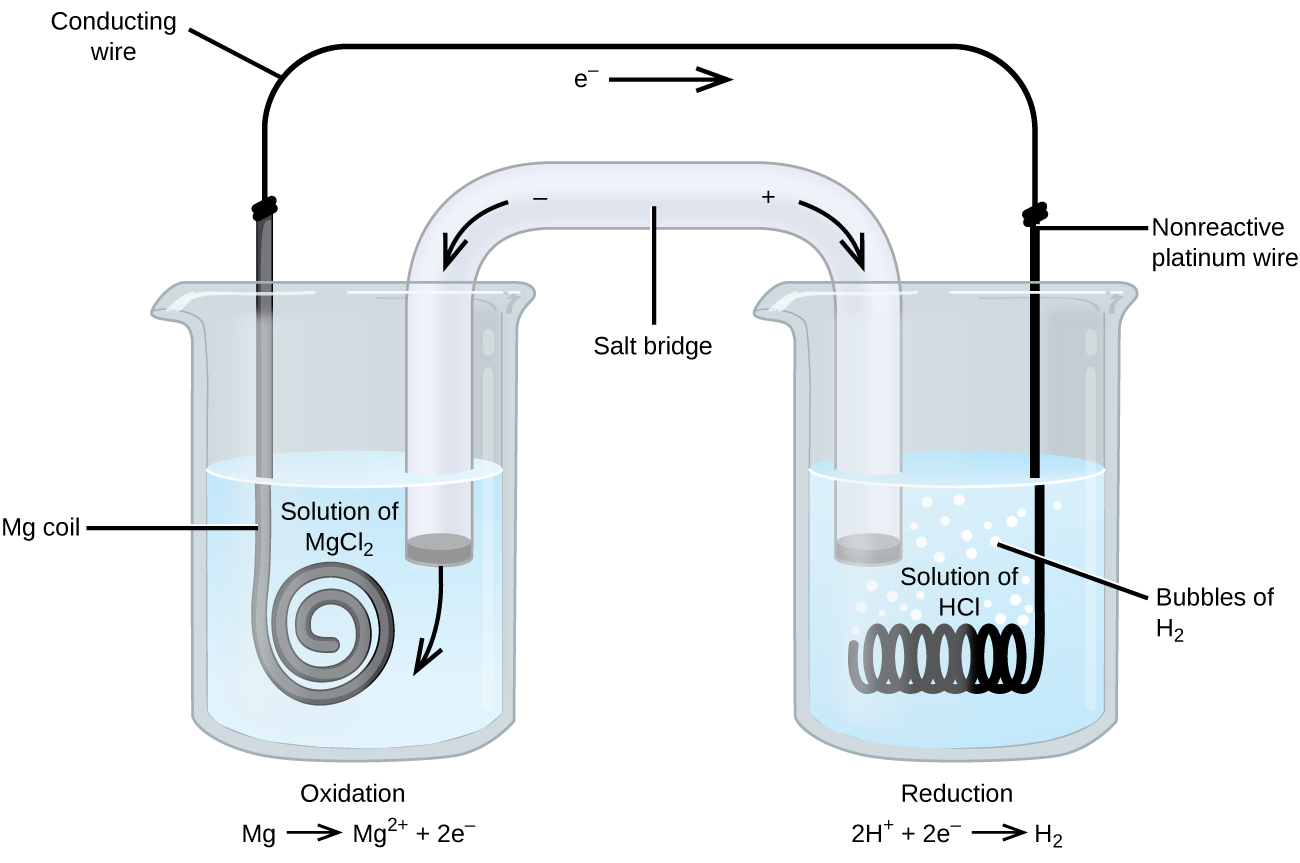






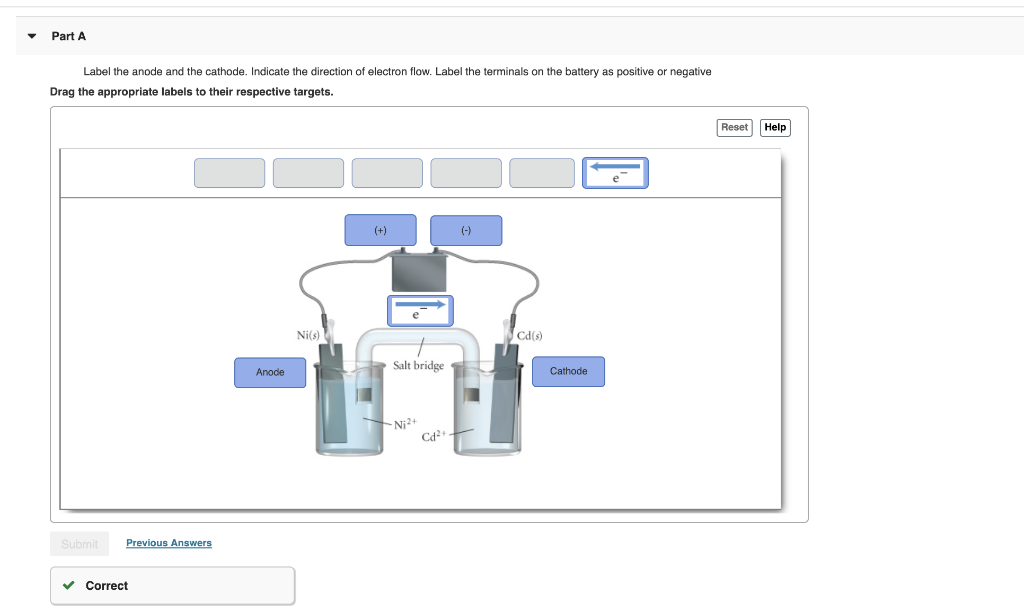
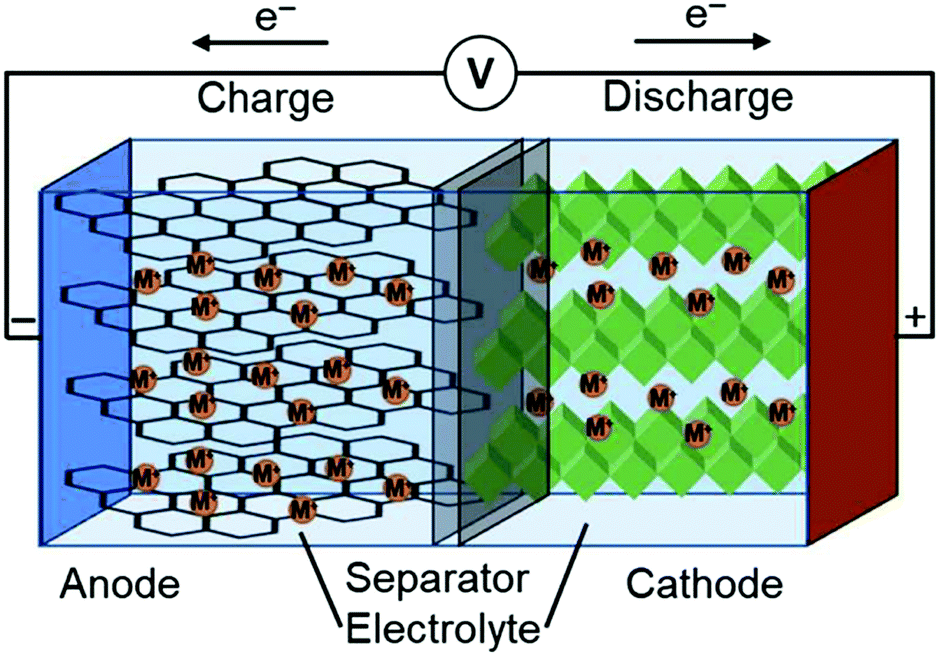



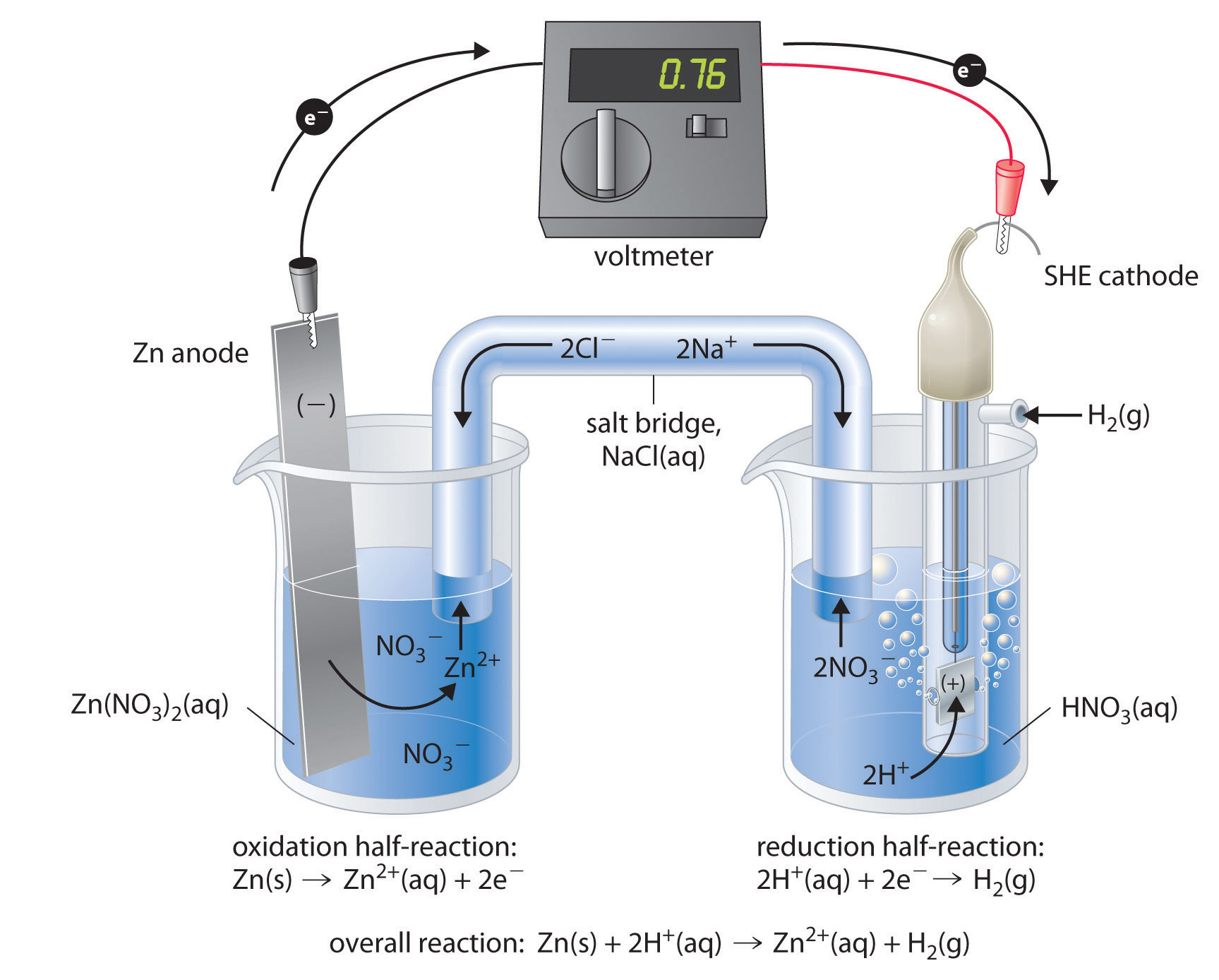

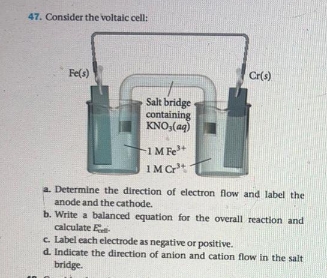


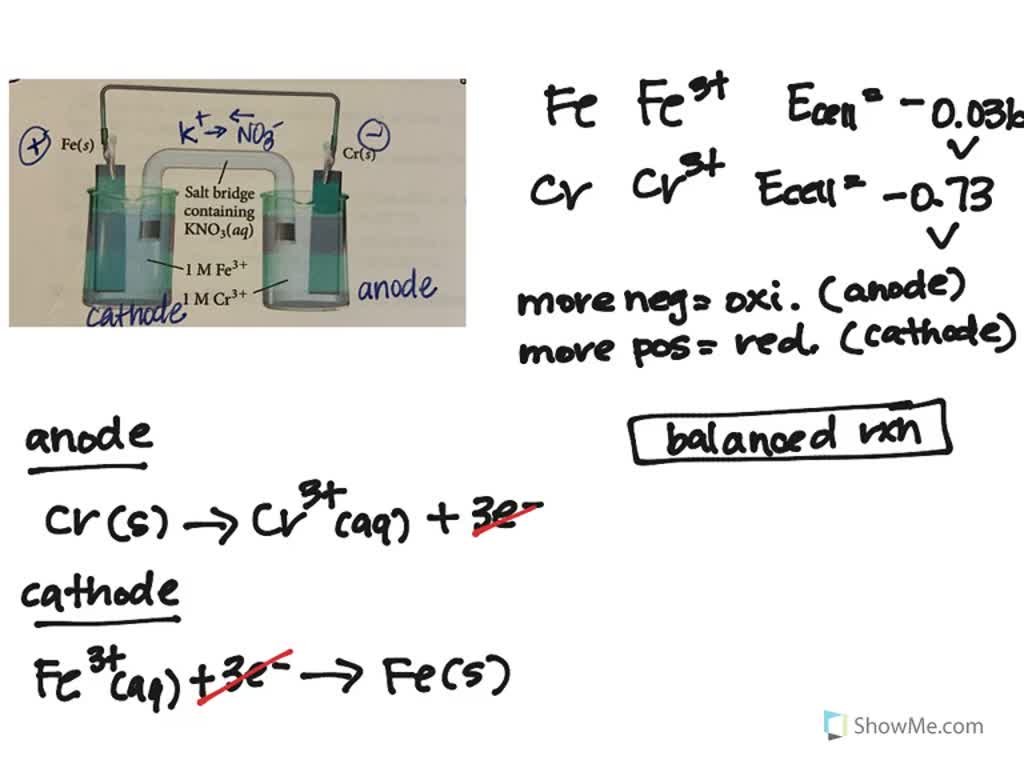


0 Response to "38 label the anode and cathode. indicate the direction of electron flow."
Post a Comment