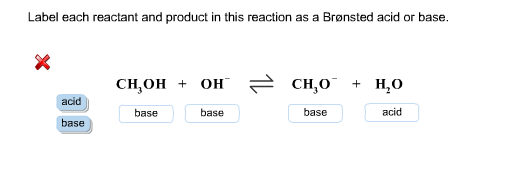
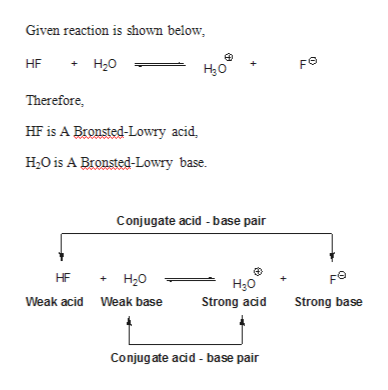
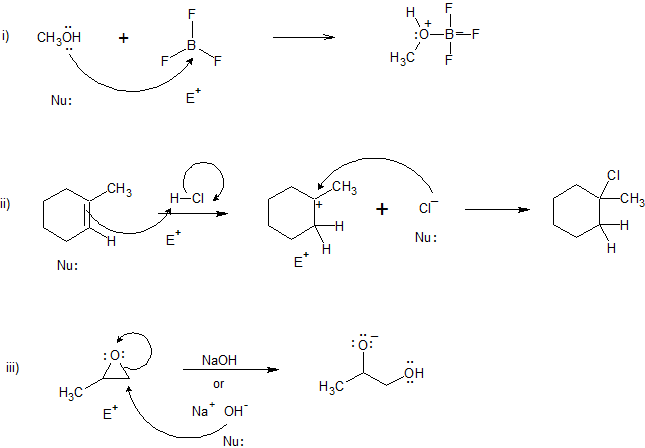
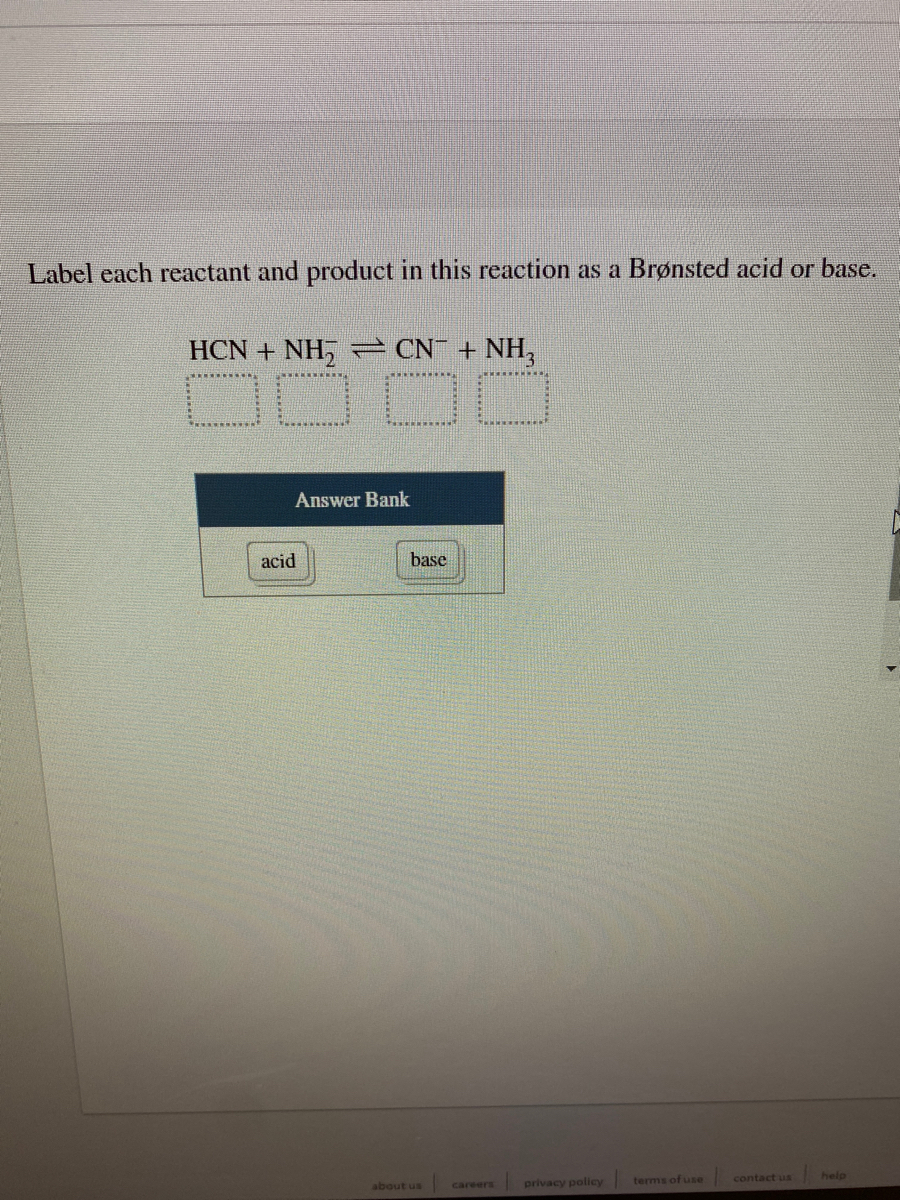
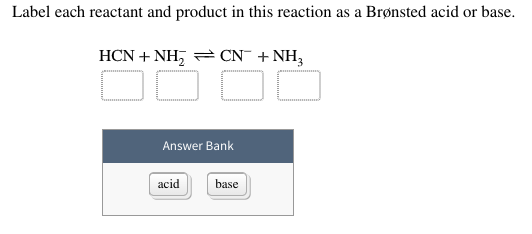
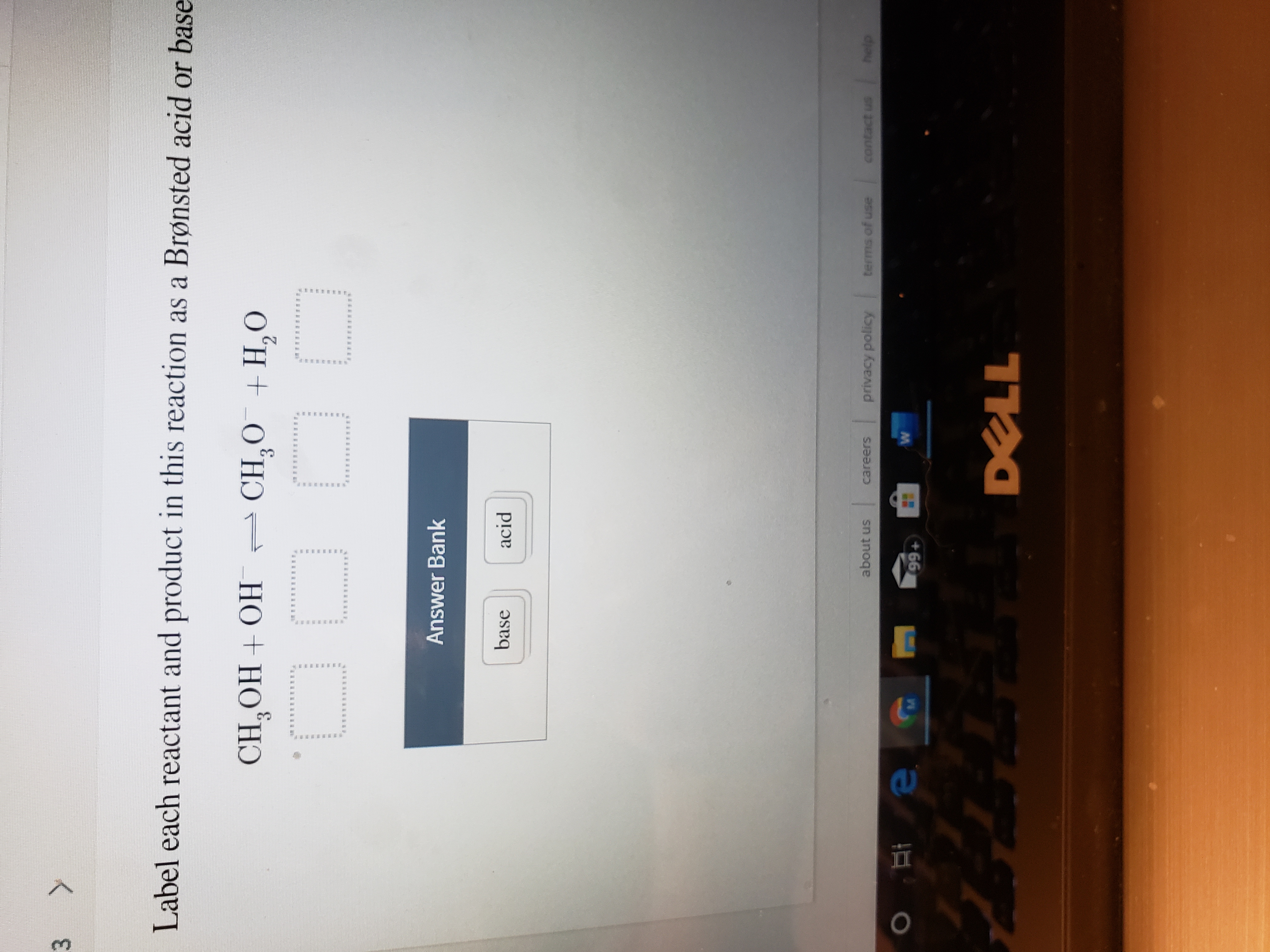
38 label each reactant and product in this reaction as a brønsted acid or base
Solved Label each reactant and product in this reaction as a These cookies may be set through our site by our advertising partners. They may be used by those companies to build a profile of your interests and show you relevant adverts on other sites. They do not store directly personal information, but are based on uniquely identifying your browser and internet... [Solved] ) In the reaction below, label each reactant as... | Course Hero Q: Label each reactant and product in the given chemical reaction. Q: Identify each reactant and product in this reaction as a Brønsted acid or base.
acid-base reaction | Definition, Examples, Formulas... | Britannica acid-base reaction, a type of chemical process typified by the exchange of one or more hydrogen ions, H+, between species that may be Almost every biological chemical process is closely bound up with acid-base equilibria in the cell, or in the organism as a whole, and the acidity or alkalinity of the...

Label each reactant and product in this reaction as a brønsted acid or base
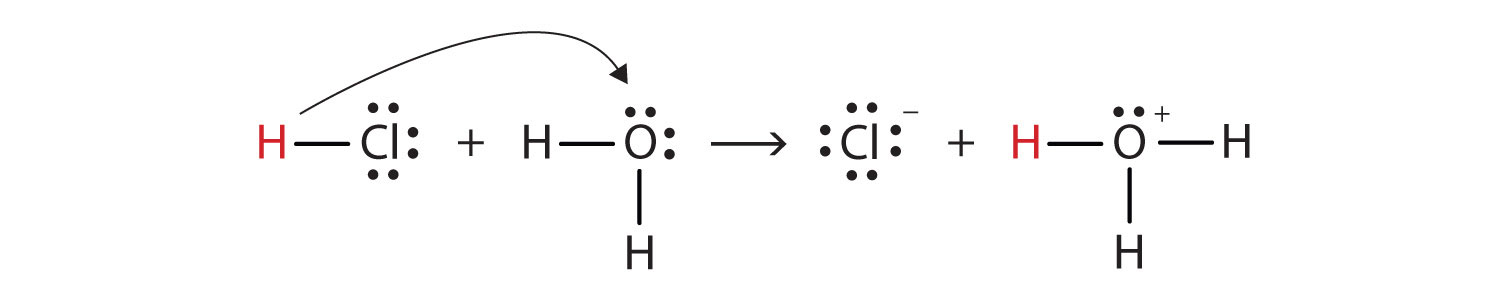
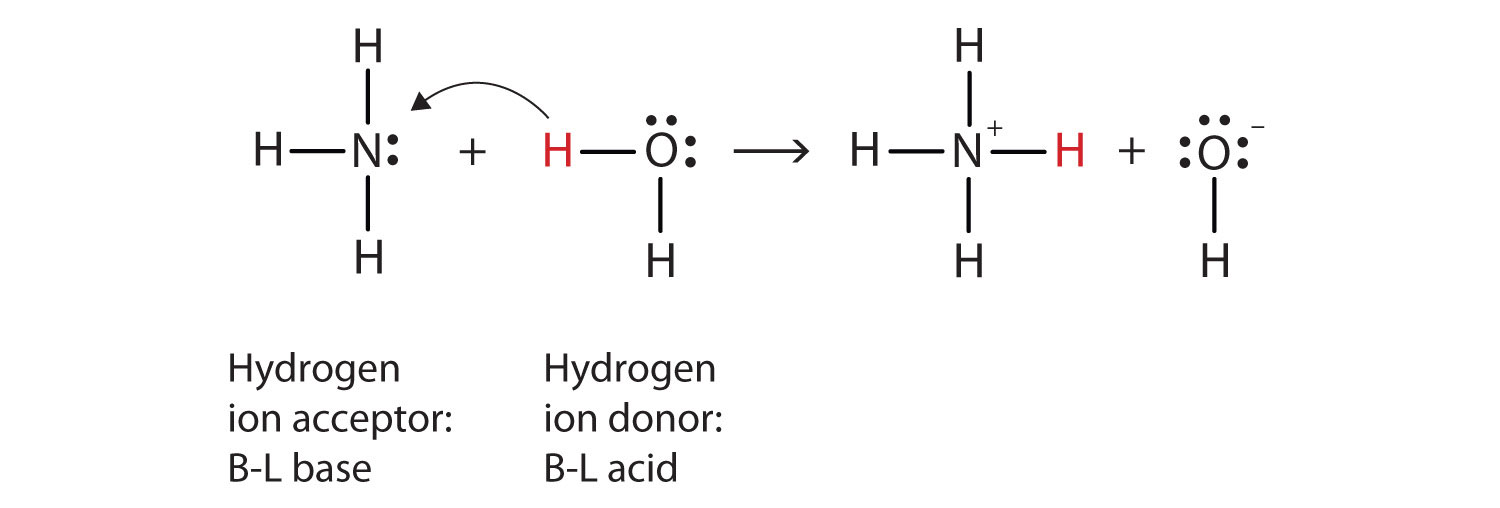
Acids, Bases, Neutralization, and Gas-Forming Reactions... Define Brønsted acids and bases. Acids that completely react in this fashion are called strong acids, and HCl is one among just a handful of common In these reactions, water serves as both a solvent and a reactant. A neutralization reaction is a specific type of acid-base reaction in which the... PDF 4.03 Acid and Bases.rtf (b) For each of the following reactions, give the formula of the acid and of its conjugate base. State the essential feature of an acid-base reaction in aqueous solution, writing an ionic equation to illustrate your answer. State the role of water in this reaction, using the Brønsted-Lowry definition. 14.1 Brønsted-Lowry Acids and Bases - Chemistry For example, consider the acid-base reaction that takes place when ammonia is dissolved in water. A water molecule (functioning as an acid) transfers The reaction between a Brønsted-Lowry acid and water is called acid ionization. For example, when hydrogen fluoride dissolves in water and ionizes...
Label each reactant and product in this reaction as a brønsted acid or base. Theories of acids and bases Ammonia reacts with water like this: This is a reversible reaction, and in a typical dilute ammonia These two are a conjugate pair. Members of a conjugate pair differ from each other by the presence The water is acting as an acid, and its conjugate base is the hydroxide ion. The hydroxide ion can... Chemical Reactivity | Examples of Brønsted Acid-Base Equilibria In an acid-base reaction, each side of the equilibrium has an acid and a base reactant or product, and these may be neutral species or ions. In all the above examples water acts as a common base. The last example ( NH3 ) cannot be measured directly in water, since the strongest base that can... Brønsted-Lowry acids and bases (article) | Khan Academy Learn about the Brønsted-Lowry definition of acids and bases. Find out how to identify the Brønsted-Lowry acid and base in a reaction and recognize the conjugate partner of each. Acids And Bases | Encyclopedia.com Acid-base indicators, such as litmus paper and other materials for testing pH, offer a means of judging these qualities in various substances. The Brønsted-Lowry acid-base theory defines an acid as a proton (H+) donor, and These two products of the reaction are called a conjugate acid-base pair...
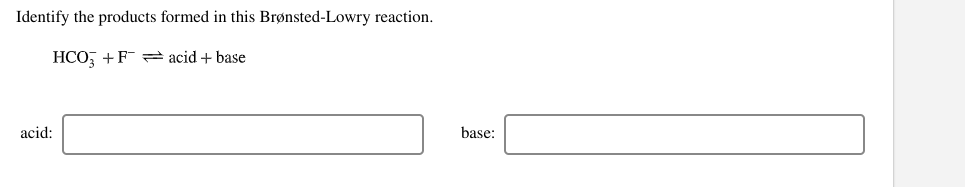
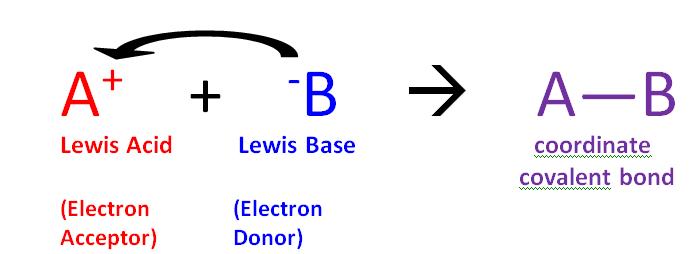
Label each reactant and product in this reaction as a Brønsted... Related questions. Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3NO2(g)+H2O(l)→2HNO3(l)+NO(g). Which reactant is in excess? Give the IUPAC name for each of the following. Calculate for the lattice energy using the diagram below. chem Flashcards | Quizlet Acids react with bases to produce salts and water. This occurs because some chemicals displace each other based on the reactivity series. Remember that as a forward reaction occurs, the reactant particles collide together to produce the products. Introduction Notice that each compound (both reactant and product) is made of a cationic and an anionic component. Acid-Base Reactions, for our purposes, there are two ways of thinking about acids and bases. The Brønsted-Lowry concept describes acid-base reactions as the transfer of a proton... label each reactant and product in this reaction as a brønsted acid... Mention the products produced when an acid reacts with a base. Give equation of an example of the reaction involved. What is this kind of reaction known as ? classify each of the following as a lewis acid or a lewis base. asked Dec 14, 2020 in Other by manish56 Expert (49.9k points).
Unit 9 Chemical Equilibrium & Acid-Base Chemistry - ppt video online... Reversible Reactions A chemical reaction in which the products can react to re-form the reactants is 24 Brønsted-Lowry Acids and Bases In a Brønsted-Lowry Acid-Base reaction, an H+ ion Water can act as a base. base acid acid base Visual Concept. 28 Polyprotic Acids Molecules with... Can an acid and base that are both in solid form react with each other? Acid-base reactions happen best in a protic medium like water, where In principle an acid and base molecule that are in contact with each other in the solid phase will Note that the titration reactions go to completion because, in each case, at least one of the reactants is a strong acid or a strong base. PDF Acidity and Basicity | Examples of Brønsted Acid-Base Equilibria In an acid-base reaction, each side of the equilibrium has an acid and a base reactant or product, and these may be neutral species or ions. In all the above examples water acts as a common base. The last example ( NH3 ) cannot be measured directly in water, since the strongest base that can... Answered: Predict the chemical formula for the… | bartleby Q: 1 Label each reactant and product in this reaction as a Brønsted acid or base.
Frontiers | The Hexameric Resorcinarene Capsule as a Brønsted Acid... Tiefenbacher et al. demostrated that C behaves as a Brønsted acid (Zhang and Tiefenbacher, 2013 The acidic behavior of C is explained by the stabilization of its conjugate-base due to the This class of products is generally obtained by means of strategies relying upon the use of Brønsted At this regard, as a model reaction for investigating the catalytic performance of C, we chose the reaction...
Acid-Base Equilibrium Part 1: How to Use... — Organic Chemistry Tutor The products in an acid base reaction are called the conjugates. Let's take for example a reaction In this reaction the ester acts as a base by accepting a proton and sulfuric acid acts as Brønsted acid by Let's look at a few species so we can see how the Ka and the pKa values compare to each other.
General Chemistry/Properties and Theories of Acids and Bases... Acids and bases are everywhere. Some foods contain acid, like the citric acid in lemons and the lactic acid in dairy. Cleaning products like bleach and ammonia are bases. Chemicals that are acidic or basic are an important part of chemistry.
Course: AP Chemistry - L. Mosier o recognize a Brønsted acid and base in a reaction and identify its conjugate. pH, pOH, [H+], and [OH o explain the relative strengths of acids such as HF, HCl, HBr, and HI or H2O, H2S, H2Se, and H2Te. o recognize that a very strong acid has a weak conjugate base and a very weak acid has...
Acid-Base Chemistry - Chemistry Encyclopedia - reaction, water... Most common acid-base reactions take place in water solutions (commonly referred to as aqueous The Brønsted-Lowry theory includes water as a reactant and considers its acidity or basicity in the Phosphoric acid has three ionizable hydrogen ions. Each stepwise ionization of phosphoric acid...
exercises - acid-base equilibria - chemistry the central science VISUALIZING CONCEPTS. 16.1 (a) Identify the Brønsted-Lowry acid and base in the reaction. Draw the Lewis structures for the reactants and products, and identify the Lewis acid and the _ 16.21 Label each of the following as being a strong base, a weak base, or a species with...
Tools & Resources: Organic Chemistry I Glossary | CliffsNotes acid-base reaction a neutralization reaction in which the products are a salt and water. Arrhenius theory a theory (limited to aqueous systems) that defines an acid as a compound that liberates competing reactions two reactions that start with the same reactants but form different products.
Introduction to Acid-Base Reactions - Master Organic Chemistry Why do some acid base reactions work, but not others? What factors determine the strengths of acids and bases? How can we determine the equilibrium lies for an We can also start thinking about the charges (or partial charges) on each reactant, and then start to depict where the electrons are going.
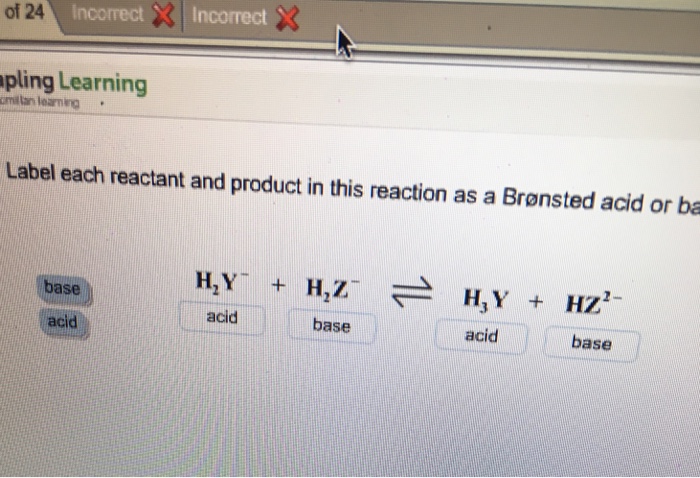
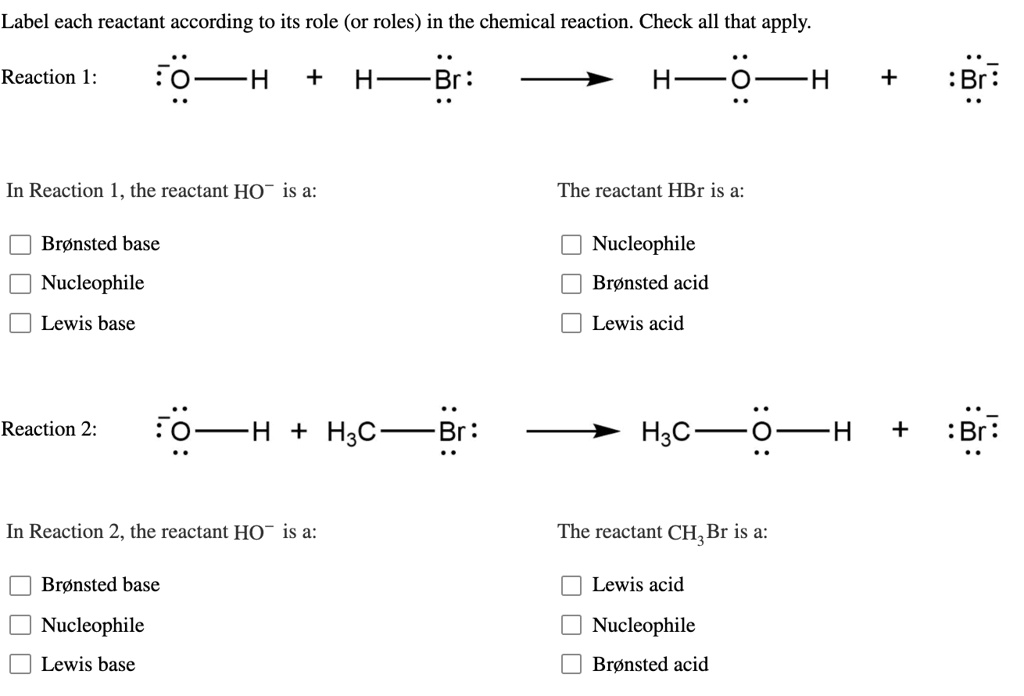
30 Label Each Reactant And Product As A Bronsted Acid Or Base 25 Acid Base Chemistry Libretexts. Ppt Chapter 19 Acids Bases And Salts Powerpoint Presentation. Label Each Reactant According To Its Role Solved Of 24 Incorrectincorrect Pling Learning Cmiltirn L. Solved Label Each Reactant And Product In This Reaction A. Add Uri Prefix Legend Below View...
Brønsted-Lowry acid-base theory - Wikipedia The Brønsted-Lowry theory (also called proton theory of acids and bases) is an acid-base reaction theory which was proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923.
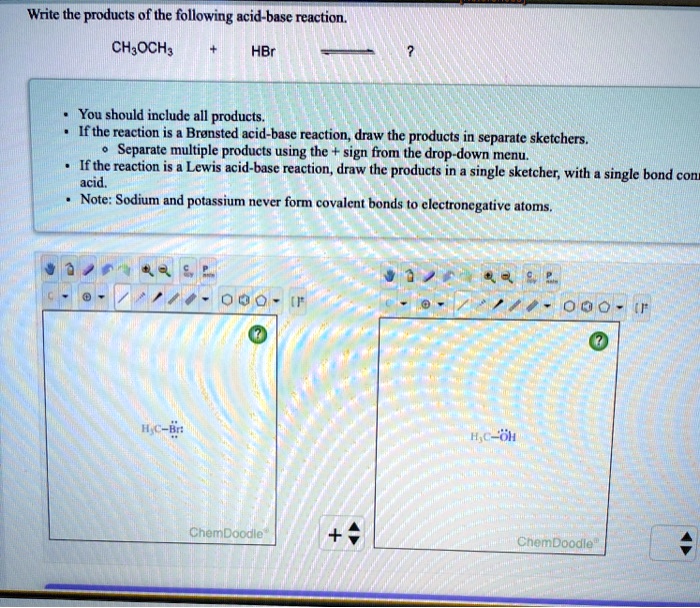
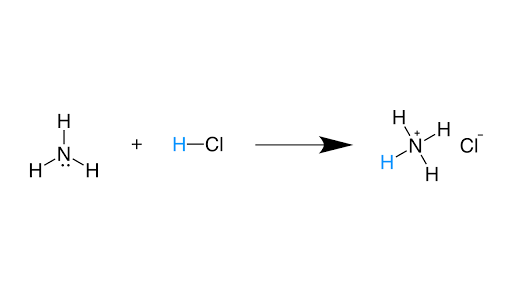
6.3. Bronsted-Lowry acids/bases | Organic Chemistry 1: An open... After a Brønsted-Lowry acid donates a proton, what remains is called the conjugate base. You have undoubtedly seen this reaction before in general chemistry. Despite its simplicity (and despite the fact that the reactants and products are inorganic rather than organic), this reaction allows us to...
10.2: Brønsted-Lowry Definition of Acids and Bases - Chemistry... Brønsted-Lowry acid-base reactions are essentially proton transfer reactions. So the Brønsted-Lowry definitions of an acid and a base classify the dissolving of HCl in water as a reaction between an acid and a base—although the Arrhenius definition would not have labeled H2O a base...
14.1 Brønsted-Lowry Acids and Bases - Chemistry For example, consider the acid-base reaction that takes place when ammonia is dissolved in water. A water molecule (functioning as an acid) transfers The reaction between a Brønsted-Lowry acid and water is called acid ionization. For example, when hydrogen fluoride dissolves in water and ionizes...
PDF 4.03 Acid and Bases.rtf (b) For each of the following reactions, give the formula of the acid and of its conjugate base. State the essential feature of an acid-base reaction in aqueous solution, writing an ionic equation to illustrate your answer. State the role of water in this reaction, using the Brønsted-Lowry definition.
Acids, Bases, Neutralization, and Gas-Forming Reactions... Define Brønsted acids and bases. Acids that completely react in this fashion are called strong acids, and HCl is one among just a handful of common In these reactions, water serves as both a solvent and a reactant. A neutralization reaction is a specific type of acid-base reaction in which the...

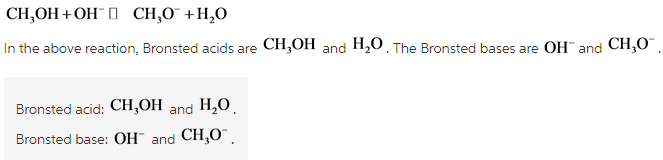

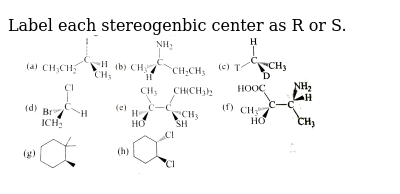
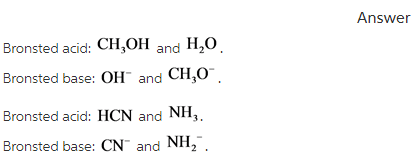



![Add URI prefix legend below view [#2472479] | Drupal.org](https://www.drupal.org/files/issues/Screen%20Shot%202015-07-02%20at%2018.05.35.png)






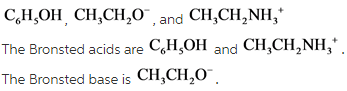
0 Response to "38 label each reactant and product in this reaction as a brønsted acid or base"
Post a Comment