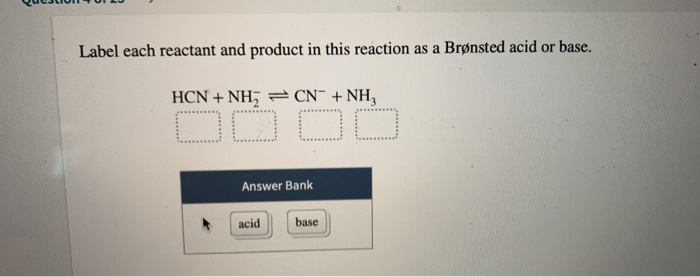
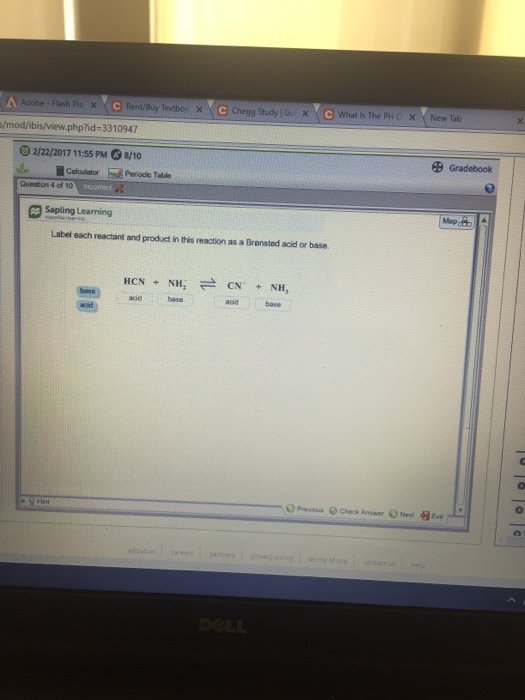
38 label each reactant and product in this reaction as a brønsted acid or base. hcn
14.1 Brønsted-Lowry Acids and Bases - Chemistry The reaction between a Brønsted-Lowry acid and water is called acid ionization. For example, when hydrogen fluoride dissolves in water and ionizes, protons are transferred from hydrogen fluoride molecules to water molecules, yielding hydronium ions and fluoride ions: (PDF) Organic Chemistry By Clayden Greeves Warren and ... Academia.edu is a platform for academics to share research papers.
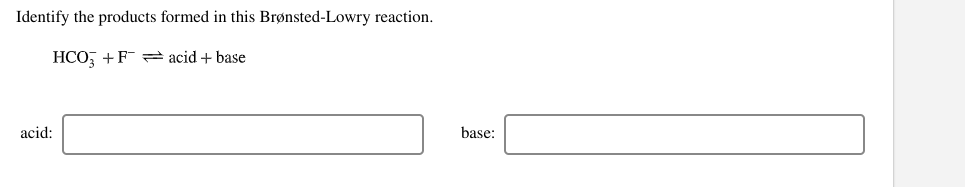
DOC Chapter 15 3. In the following reactions, name each of the reactants as a Lewis acid or base. a. Cu2+ + 4NH3 ( Cu(NH3)42+ b. Zn(OH)2 + 2OH- ( Zn(OH)42- 4. In the following reaction, name each of the reactants as a Brønsted-Lowry acid or base, and give the conjugate of each. HCO3- + H2O ⇌ H2CO3 + OH- 5.
Label each reactant and product in this reaction as a brønsted acid or base. hcn
Answered: 1 Label each reactant and product in… | bartleby 1 Label each reactant and product in this reaction as a Brønsted acid or base. HCN+NH2−↽−−⇀CN−+NH3 check_circle Expert Answer star star star star star 1 Rating Want to see the step-by-step answer? See Answer Check out a sample Q&A here. Want to see this answer and more? PDF Chapter 2 Hw Solutions Acid-base Reactions 3OH) is "amphoteric", meaning it can act as both a Brønsted acid and a Brønsted base. Give the likely products of these reactions, and indicate whether methanol is acting in each as an acid or base. (Base) CH 3OH + HCl ! CH 3OH 2 ++ Cl− (Acid) CH 3OH + NaNH 2! CH 3ONa + NH 3(Recall CH 3ONa means CH 3O −Na+) HN H H a. H+CCCH3 Acid-Base Concepts - New Mexico State University An amphiprotic species is a species that can act as either an acid or a base (it can lose or gain a proton), depending on the other reactant. For example, HCO 3- acts as an acid in the presence of OH - but as a base in the presence of HF. Anions with ionizable hydrogens, such as HCO 3- , and certain solvents, such as water, are amphiprotic.
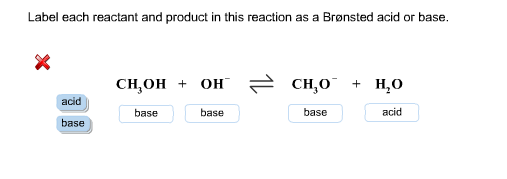
Label each reactant and product in this reaction as a brønsted acid or base. hcn. Identify the products formed in this Brønsted-Lowry reaction. Explanation: According to Bronsted-Lowry, acids are the species which donate hydrogen ions to another specie in a chemical reaction. Bases are the species which accept a hydrogen ion upon chemical reaction. For example, Here, the products formed in this Bronsted-Lowry reaction are and . Chemistry Question with reactant and product please? Label each reactant and product in this reaction as a Bronsted acid or base. HCN + NH2 -> <- CN + NH3 options for reach one is either base or acid. Thank You! 2 Answers A Bronsted acid is a proton donor (loses an H+ in the reaction) and a Bronsted base is a proton acceptor (gains an H+ in the reaction) Chem 1412 Hw 10 Flashcards - Quizlet H2PO4- --> HPO42- NH4+ --> NH3 4. Label each reactant and product in this reaction as a Bronsted acid or base. HCN - Acid NH2- - Base CN- - Base NH3 - Acid 5. Consider three generic acids with the following relative strengths: HX > HY > HZ Rank the strengths of their conjugate bases. Strongest Base Z- Y- X- Weakest Base 6. OneClass: Label each reactant and product in this reaction ... Label each reactant and product in this reaction as a Brønsted acid or base. HCN + NH 2- >><< CN - + NH 3 Answer + 20 Watch For unlimited access to Homework Help, a Homework+ subscription is required. Coleen Amado Lv10 22 Nov 2020 Unlock all answers Get 1 free homework help answer. Already have an account? Log in
Solved Label each reactant and product in this reaction as ... See the answer Label each reactant and product in this reaction as a Brønsted acid or base. HCN+NH−2−⇀↽−CN−+NH3HCN+NH2−↽−−⇀CN−+NH3 Expert Answer 100% (22 ratings) HCN + NH2- CN- + NH3 HCN is Bronsted a … View the full answer Previous question Next question Solved > Question 2. The alcohol product contains two ... Question Label each reactant and product in this reaction as a Bronsted acid or base. HCN + NH^- 2 rightleftharpoons CN^- + NH3 base acid... Question Label each reactant and product in this reaction as a Bronsted acid or base. Write the balanced chemical equation for the reaction of the weak... Page 1076 of 2502 for Chemistry Answers, Learning Aids ... Question Label each reactant and product in this reaction as a Bronsted acid or base. Write the balanced chemical equation for the reaction of the weak acid HCN with water. Include the phase of each species. Consider three generic acids with the following relative stengths: HX > HY > HZ Rank. PDF Sample Exercise 16.1 Identifying Conjugate Acids and Bases In both cases identify the conjugate acid- base pairs. When lithium oxide (Li. 2. O) is dissolved in water, the solution turns basic from the reaction of the oxide ion (O. 2 -) with water. Write the reaction that occurs, and identify the conjugate acid- base pairs. Answer: O. 2- (aq) + H. 2. O(l) →OH - (aq) + OH - (aq). OH - is ...
HCN(aq) + SO4-2(aq) HSO4-(aq) + CN -(aq) a. What is the ... Calculate the pH of 0.20 M NaCN solution. NaCN ---> Na+ + CN- CN- + H20+ ---> HCN+ + OH- Initial conc. of CN- = 0.20 mol/L change = -x equillibrium = 0.20-x HCN equill. = +x OH equill. = 1x10^-7+x Ka= 6.2 x 10^-10 Kb = KW/Ka = Chemistry Identify the acid-base pairs in the following reactions HSO4^1- (aq) + OH^1- SO4^2- (aq) + H2) (I) Chemistry Brønsted-Lowry Acids and Bases | Chemistry base +proton ⇌ conjugate acid OH - +H + ⇌ H 2 O H 2 O +H + ⇌ H 3 O + NH 3 +H + ⇌ NH 4 + S 2− +H + ⇌ HS - CO 3 2− +H + ⇌ HCO 3 - F - +H + ⇌ HF In these two sets of equations, the behaviors of acids as proton donors and bases as proton acceptors are represented in isolation. Acid/Base Practice Problems Answers The acid-base reaction in the gas phase is: B(g) + H + (g) → HB + (g)Steric effects have a small role in this reaction since the gas phase hydrogen ion is so small. Thus, the electron donating ability of the methyl groups primarily influences the base strength: increasing the number of methyl groups increases the electron density in the nitrogen lone pair, leading to the increased base strength. (PDF) general-chemistry.pdf | Sumit Banerjee - Academia.edu Academia.edu is a platform for academics to share research papers.
PDF TABLE OF CONJUGATE ACID-BASE PAIRS Acid Base Ka (25 C) TABLE OF CONJUGATE ACID-BASE PAIRS Acid Base K a (25 oC) HClO 4 ClO 4 - H 2 SO 4 HSO 4 - HCl Cl- HNO 3 NO 3 - H 3 O + H 2 O H 2 CrO 4 HCrO 4 - 1.8 x 10-1 H 2 C 2 O 4 (oxalic acid) HC 2 O 4 - 5.90 x 10-2 [H 2 SO 3] = SO 2 (aq) + H2 O HSO
Answered: For the chemical equations shown below,… | bartleby Science Chemistry Q&A Library For the chemical equations shown below, label each reactant as either acid or base, and each product as either conjugate acid or conjugate base according to the Brønsted-Lowry definition. Drag each label to the appropriate target. > View Available Hint(s) Reset Help conjugate acid conjugate base acid base HPO, (aq) + H,0(1) = H,PO, (aq) + OH (aq) HPO, (aq) + H,0 ...
32 Label Each Reactant And Product In This Reaction As A Brønsted Acid Or Base. Hcn - Labels For ...
Solved Label each reactant and product in this reaction as ... Label each reactant and product in this reaction as a Bronsted acid or base. Classify each of the following reactants and products as an acid or base according to the Bronsted theory CF3COOH + H2,0 H3O+ + CF3COO- This problem has been solved! See the answer O. Chem Show transcribed image text Expert Answer 100% (87 ratings)
HCN(aq) + SO4-2(aq) HSO4-(aq) + CN -(aq) a. What is the ... Identify the Bronsted-Lowry acid in the following reaction. H2O (l) + HCO31- (aq) ¨ H3O+ (aq) + CO32- (aq) chem 2 indictae the reactant that is a bronsted lowry acid. HCN (aq) +H2O (l)---> H3O+ (aq)=CN- (aq) HCN CN- H20 H30 i think it is HCN the weak acid substance which acts as a proton (H+) donor and CN the weak base? You are right.
PDF Test2 ch17a Acid-Base Practice Problems 2. In the Brønsted-Lowry definition of acids and bases, a base _____ a. is a proton donor. d. breaks stable hydrogen bonds. b. is a proton acceptor. e. corrodes metals. c. forms stable hydrogen bonds. 3. In the following reaction in aqueous solution, the acid reactant is _____ and its conjugate base product is _____. CH 3COOH + NH 3 CH 3COO
Bronsted Lowry Theory of Acids and Bases - ThoughtCo The Brønsted-Lowry acid-base theory (or Bronsted Lowry theory) identifies strong and weak acids and bases based on whether the species accepts or donates protons or H +. According to the theory, an acid and base react with each other, causing the acid to form its conjugate base and the base to form its conjugate acid by exchanging a proton.
Chapter 15, Acids and Bases Video Solutions ... - Numerade a) Consider the hydrated aluminum ion Al(H2O)63 + 6. as a Brønsted-Lowry acid. Write the chemical equation in which this ion loses a proton in a reaction with ammonia, NH3. Identify the conjugate acids and bases in this reaction. b) Ethanethiol, CH3CH2SH, is a malodorous compound present in petroleum.
PDF NGSS Regents Chemistry - pendleton.k12.ky.us In the forward reaction below, HCl acts as the acid, and OH-acts as the base. Example: HCl + OH- Cl-+ H 2O In the following equations, draw brackets between conjugate acid-base pairs and label each species as a Bronsted-Lowry acid or base, and answer the question. 1) According to the Bronsted-Lowry theory, what does H3O

0 Response to "38 label each reactant and product in this reaction as a brønsted acid or base. hcn"
Post a Comment