38 label the reactants products and intermediates
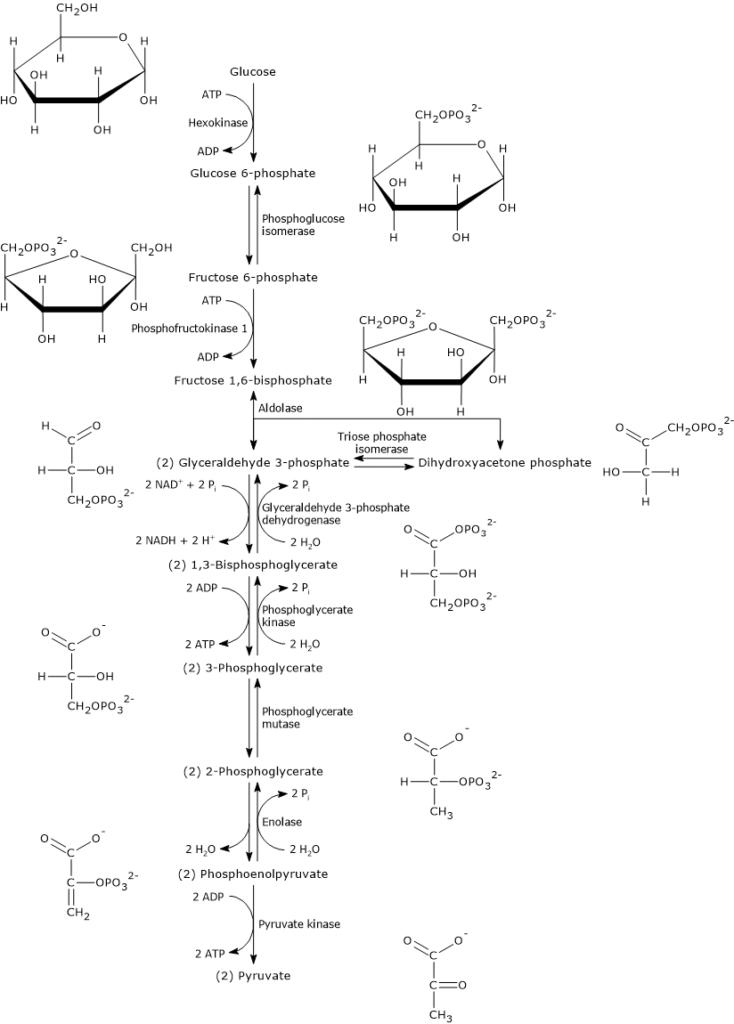
The Reactants And Products Of Cellular Respiration ... Cellular respiration is the process responsible for converting chemical energy, and the reactants/products involved in cellular respiration are oxygen, glucose (sugar), carbon dioxide, and water. While the exact steps involved in cellular respiration may vary from species to species, all living organisms perform some type of cellular respiration. Part A Select the approximate reaction ... - Course Hero Part B Label the parts that represent the reactants, products, and transition state. Also label the activation energy and heat of this reaction. Drag the appropriate labels to their respective targets. ANSWER: Correct Reactants are present initially before the reaction has started.
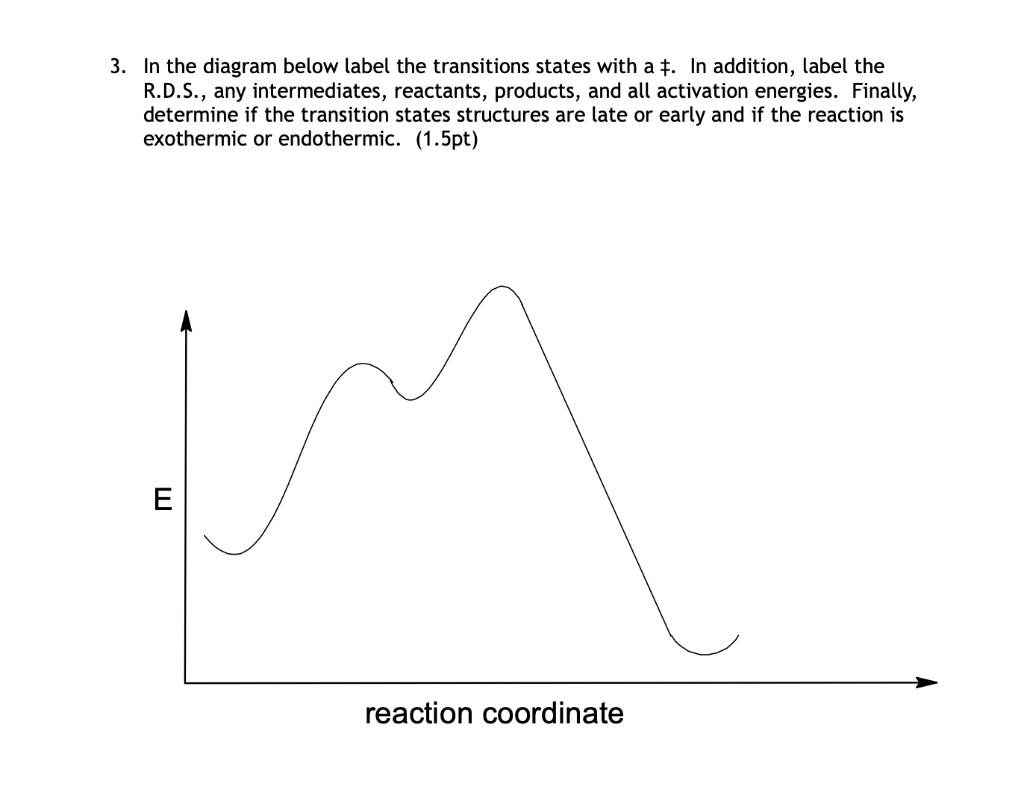
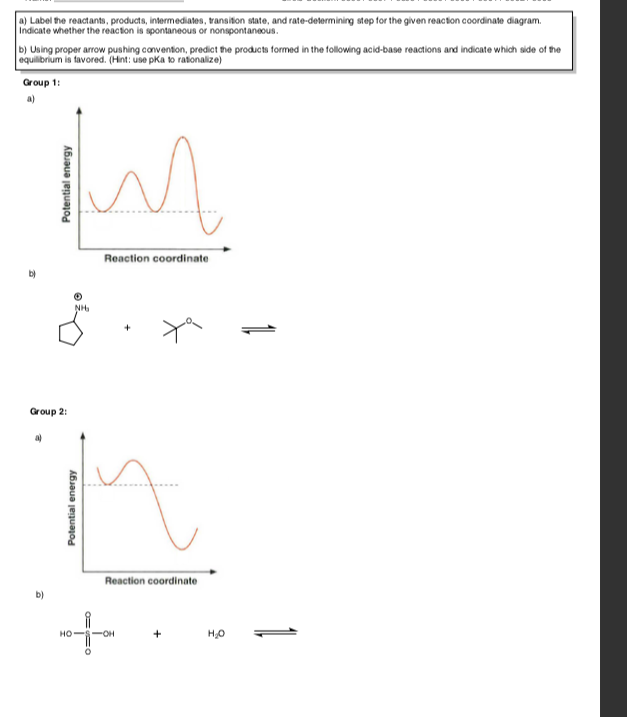
test 2 worksheets.pdf - Chapter 05 Worksheet 01 Draw the ... Chapter 05 Worksheet 01 Draw the reaction energy diagram, and label the products, reactants, transition states, and intermediates. 1. • 3-Step reaction • 2 nd step is Rate-Determining-Step • 3 rd step is faster than the 1 st • 1 st step is endothermic • 2 nd step is exothermic • 3 rd step is exothermic • The overall reaction is ...

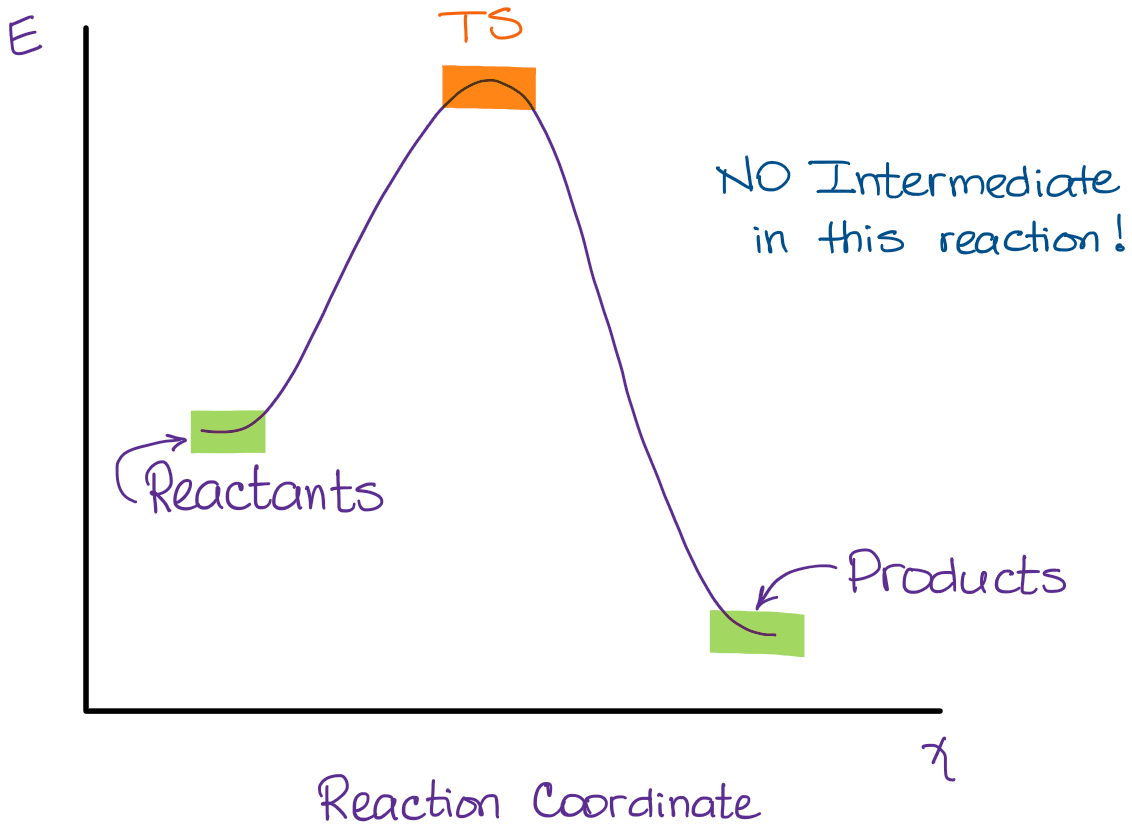
Label the reactants products and intermediates
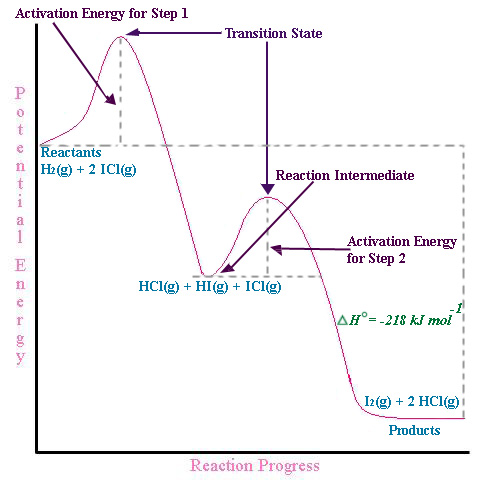
Solved - Part A Label the reactants, the products, the ... Question: - Part A Label the reactants, the products, the intermediates and the transition states, Drag the appropriate labels to their respective targets. Note: not all targets will be used. Reset Help reactants intermediate transition state products Is the overall reaction endothermic or exothermic? Chapter 14: Practice Flashcards - Quizlet Potential energy of products Draw a graph showing the reaction pathway for an overall exothermic reaction with two intermediates that are produced at different rate. On you graph indicate the reactants, products, intermediates, transition states, and activation energies. Label the reactants, products, intermediates, and ... Label the reactants, products, intermediates, and transition states. Identify the rate-determining step Drag the appropriate labels to their respective targets. Reset Help 1 3 N Group 2 Group 2 Group 2 3 Group 2 E reactants Group 2 products Group 2 Group 2 intermediate The rate-determining step is Group 1 transition state Reaction progress ...
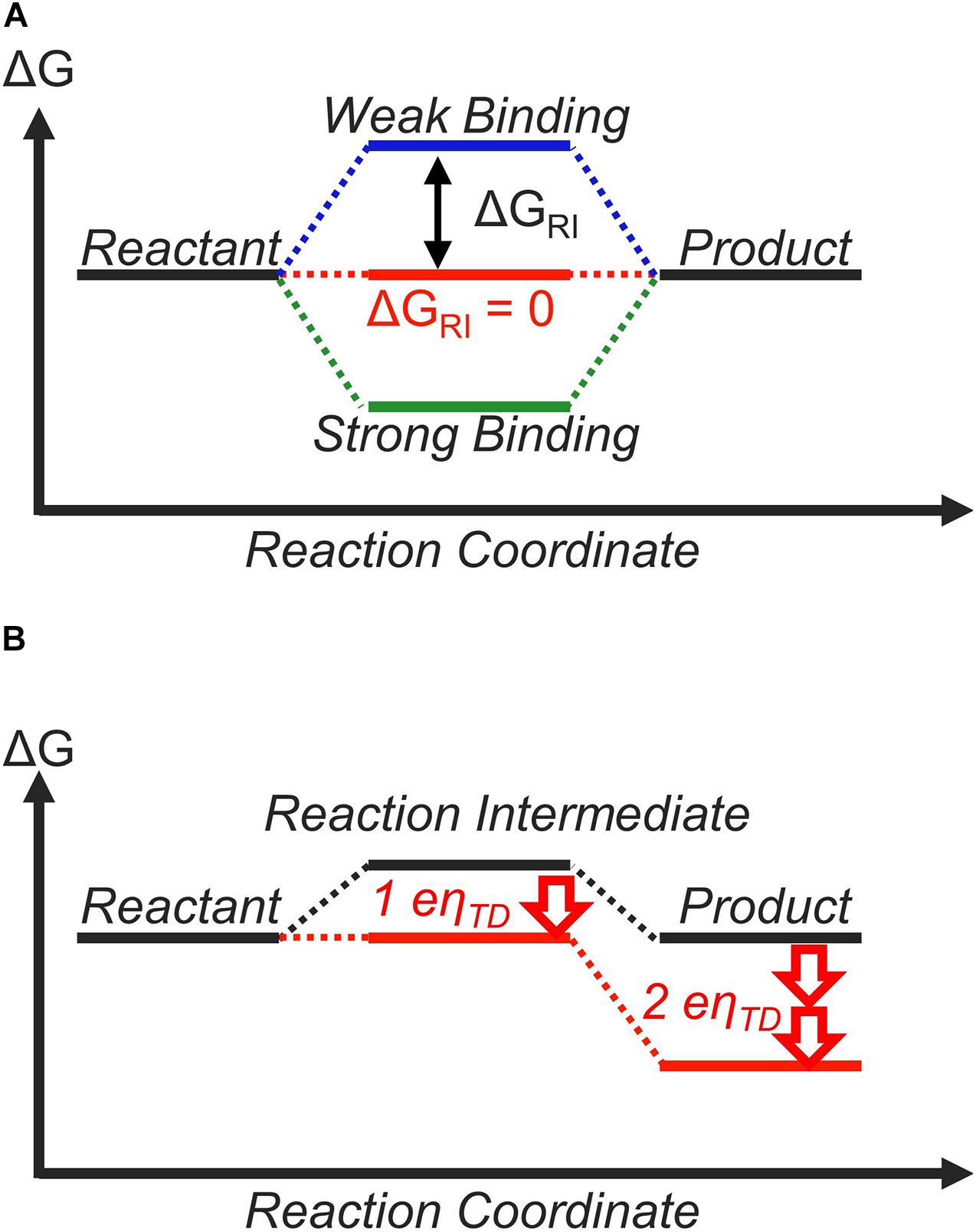
Label the reactants products and intermediates. PDF CHM 221: Organic Chemistry II - North Central College bromopentane. Label the positions for all reactants, intermediates and products. State which alkyl bromide would be the major product and why. [Notice! which curve has the higher energy carbocation intermediate and higher energy first transition state. Since these two possible reactions would be competing with each ENERGY TRANSFORMATIONS Flashcards - Quizlet Transition-state intermediates have higher energies than the reactants and/or products because a) the transition state is further along the time course of the reaction. b) energy has been used to supply the enzyme to the reaction. c) the chemical bonds of the reactants have been stretched and stressed to near breaking. [SOLVED] 6. Based on the mechanism proposed ... - CoursePivot Based on the mechanism proposed in the 'Data Sheets' section of your lab manual, sketch the reaction profile. Properly label the profile and include the identification of mechanistic steps, location of the activation energies (values not required), Lewis structures of the reactants, intermediates and products in the appropriate locations. (6 marks) organic chemistry - Can the reaction intermediate be lower ... I am confused because the solutions manual to one of the problems gives me an energy diagram with an intermediate that is of lower energy than both the reactants (but not the products), and labels the overall activation energy as the energy change between the intermediate state and the highest-energy transition state.
OneClass: 95. Consider this energy diagram: Energy ... Consider the reaction coordinate diagram below. Reaction Coordinate How many steps are in this reaction? Which step is the rate-determining step? Label the reactants, products, enthalpy, and all transition states, activation a. b. c. energies, and intermediates. d. Is this reaction exo- or endothermic? Label the reactants, products, and intermediates ... Label the reactants, products, and intermediates. - 3312171 LilmayRaegan LilmayRaegan 03/31/2017 Chemistry High School answered Label the reactants, products, and intermediates. 1 See answer Advertisement PDF Chapter 10 - Organohalides - Iowa State University Label the chirality centers in the monochlorination products of methylcyclopentane with an asterisk. ... 1° character, and thus, is less stable. The allylic radical intermediate for products A and B is 2° ... Provide structures for the reactants, intermediates, or products, as indicated, in the following reactions. Draw SOLVED:Draw a reaction coordinate diagram for a two-step ... Label the reactants, products, intermediates, and transition states. Answer A reaction in which the free energy of reactants is greater than free energy of products is termed as exergonic reaction. Reaction in which free energy of reactants is lower than the free energy of products is termed as endergonic reaction. View Answer Discussion
SOLVED:Consider this energy diagram: a. How many ... for a problem. 95. We got a reaction. Diagram were energy diagram. That kind of looks like this. You start with the when you go on to pumps before you get to the brothers. Okay, so this is energy on the y axis, and this is your reaction, but Okay, so part A is asking how many elementary steps you have. So, um, the easiest way I think to figure out the number of elementary Stubbs is to find how ... Answered: Draw a reaction coordinate diagram for… | bartleby Solution for Draw a reaction coordinate diagram for a fast, stepwise, exothermic reaction. Label reactants, products, intermediate(s), transition state(s),… GC 14.1 Flashcards | Quizlet Draw a graph showing the reaction pathway for an overall exothermicreaction with two intermediates that are produced at different rates.On your graph indicate the reactants, products, intermediates,transition states, and activation energies The reaction is exothermic because the energy of products is lower than the energy of reactants. PDF Chapter 06 Worksheet 01 Give the products of the following ... Give the products of the following reactions. Be sure to indicate the proper stereochemistry when appropriate. 1. C CH3 Br H D CH3OH CH3O 2. C CH3 Br CH3 D CH3OH Heat 3. C Br Et Me CH2CH3 CH3CH2OH Heat 4. CC Br H H CH3 Et D t-bu-OH t-bu-O 5. H OTos CH3CH2S-Na+ DMSO . 6. Cl CH3CH2O ... and label the products, reactants ...
CHEM 1B PCC intermediate or Catalyst Drawing a Reaction ... Identifying reactants, products, catalyst and intermediates in a reaction mechanism is vital in understanding organic chemistry. Watch the video below and then draw the reaction diagram for the mechanism presented in the video and clearly label all the reactants, products, intermediates and catalysts in the reaction diagram.
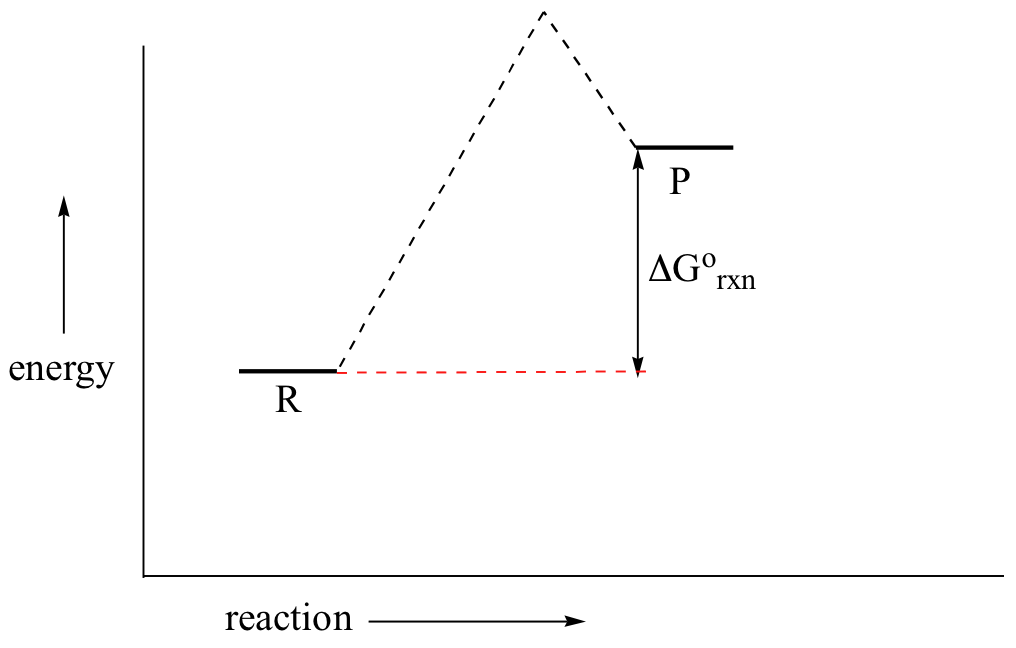
[Solved] Draw an energy diagram for a one-step reaction ... Draw an energy diagram for a one-step reaction with Keq < 1. Label the parts of the diagram corresponding to reactants, products, transition state, ΔG°, and ΔG++.
How can I draw activation energy in a diagram? | Socratic 2. Draw and label two short horizontal lines to mark the energies of the reactants and products. 3. Draw the energy level diagram. There must be a hump in the curve to represent the energy level of the activated complex. 4. Draw and label the activation energy. Draw a horizontal line from the highest part of the curve towards the vertical axis.
(Get Answer) - 1. On The Energy Versus Reaction Progress ... 1. On The Energy Versus Reaction Progress Diagram Below, Label The Reactants And Products, Any Transition States, And Reaction Intermediates, The Change In Enthalpy For The Overall Reactions, And Any Activation Energies. Potential Energy Reaction Progress Is The Reaction Exothermic Or Endothermic?...
SOLVED:Draw a reaction coordinate diagram for a two-step ... Products, intermediates and chronic transition is start stairs. So this is the reaction co ordination diagram in which this is for free energy, the bikes and X axis for the the reaction coordinates. And here the car off reactant and speak at the transition estate One and another peek at the transition estate to this is the intermediate on.
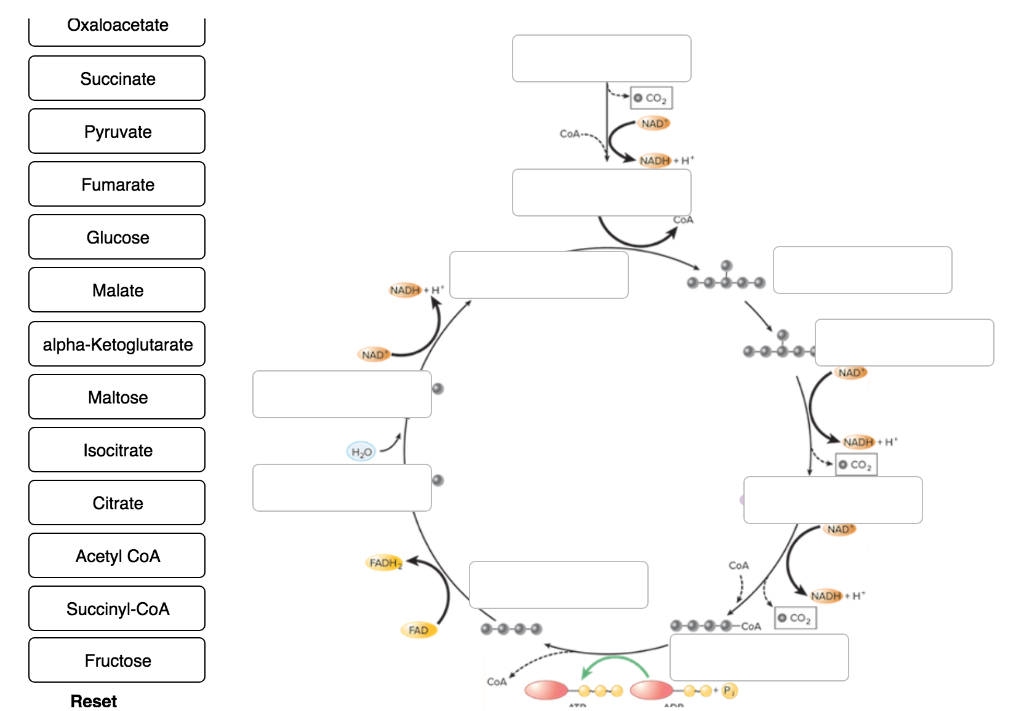
16 - SHORT ANSWERS - Citric Acid Cycle Flashcards - Quizlet Describe the enzymes, cofactors, intermediates, and products the pyruvate dehydrogenase complex. ... Show the structures of the reactants and products for two of the four redox reactions in the citric acid cycle. Indicate where any cofactors participate, and label the reactants, products, and cofactors as oxidants or reductants in the reaction.
Label the reactants, products, intermediates, and ... Label the reactants, products, intermediates, and transition states. Identify the rate-determining step Drag the appropriate labels to their respective targets. Reset Help 1 3 N Group 2 Group 2 Group 2 3 Group 2 E reactants Group 2 products Group 2 Group 2 intermediate The rate-determining step is Group 1 transition state Reaction progress ...
Chapter 14: Practice Flashcards - Quizlet Potential energy of products Draw a graph showing the reaction pathway for an overall exothermic reaction with two intermediates that are produced at different rate. On you graph indicate the reactants, products, intermediates, transition states, and activation energies.
Solved - Part A Label the reactants, the products, the ... Question: - Part A Label the reactants, the products, the intermediates and the transition states, Drag the appropriate labels to their respective targets. Note: not all targets will be used. Reset Help reactants intermediate transition state products Is the overall reaction endothermic or exothermic?
:max_bytes(150000):strip_icc()/GettyImages-944092980-819822c860ac4b1bb4c4b3a5170878ac.jpg)





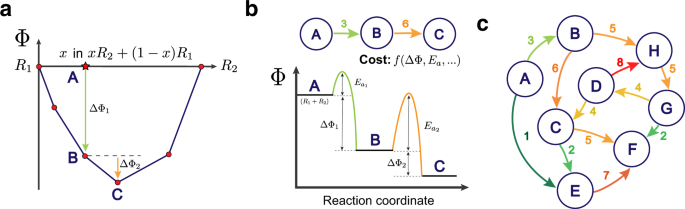
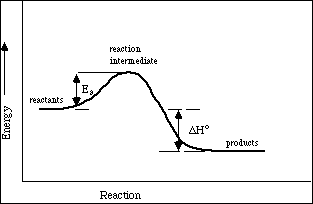


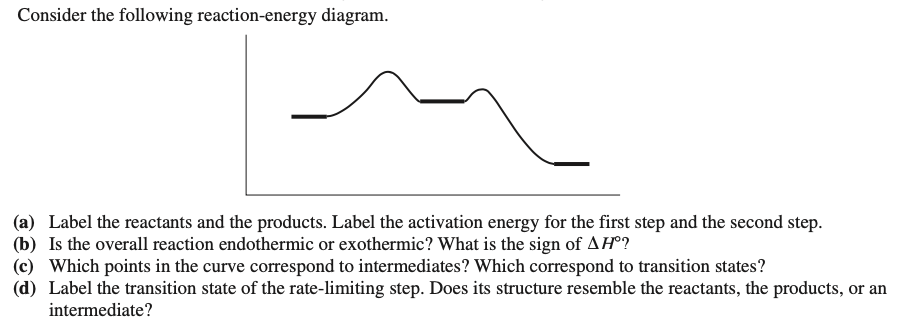


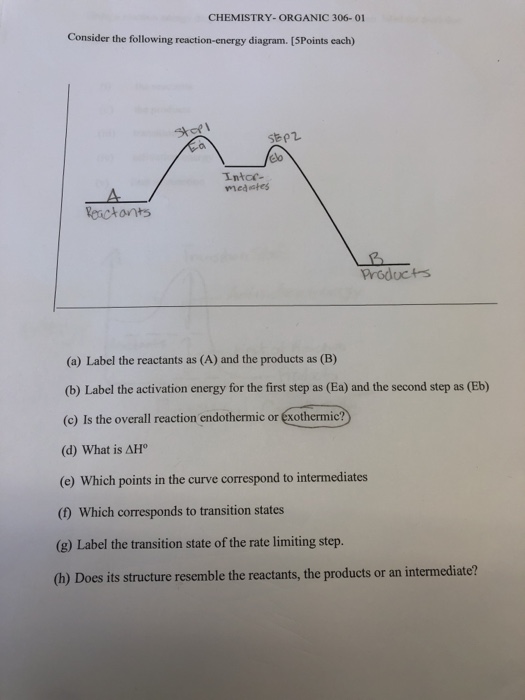





0 Response to "38 label the reactants products and intermediates"
Post a Comment