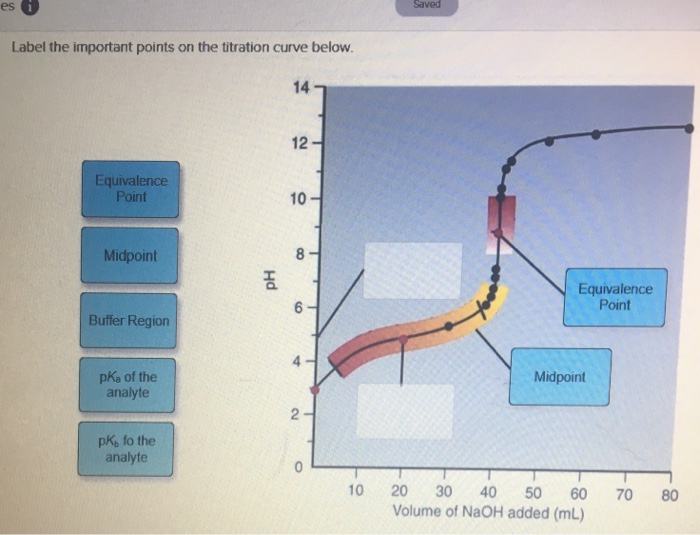
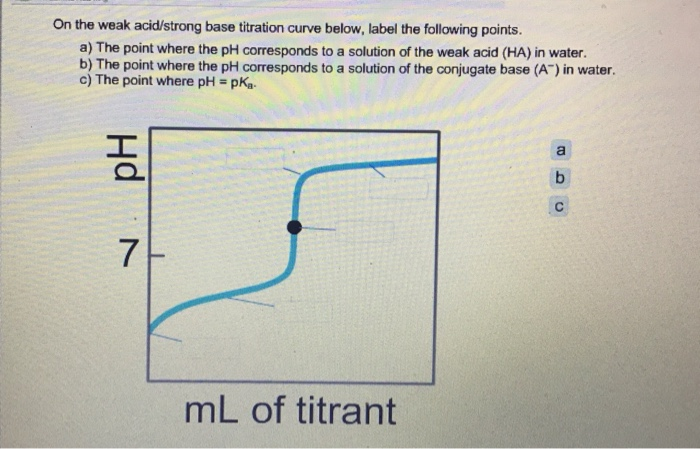
40 label the important points on the titration curve below.
Solved Label the important points of the titration curve ... Chemistry questions and answers. Label the important points of the titration curve below. 14 - Equivalence Point Midpoint Hd Buffer Region pk of the analyte pkn of the analyte 10 20 30 40 50 60 Volume of NaOH added (mL) 70 80. Acid-Base Titrations | Chemistry - Lumen Learning A titration curve is a graph that relates the change in pH of an acidic or basic solution to the volume of added titrant. The characteristics of the titration curve are dependent on the specific solutions being titrated. The pH of the solution at the equivalence point may be greater than, equal to, or less than 7.00.
Titration curves and acid-base indicators - Khan Academy For a strong acid-strong base titration, the equivalence point is at pH 7. The pH range of phenolphthalein is about 8.3 to 10.0, but the titration curve is so steep at the equivalence point that phenolphthalein makes a good indicator. For a strong base-weak acid titration, the equivalence point is probably near pH 9.
Label the important points on the titration curve below.
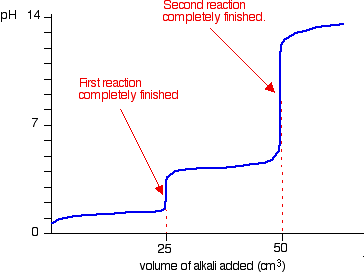
Titration - basics , curve, molarity equation & more Equivalence point titration mode (EQP): The equivalence point is the point at which the analyte and the reagent are present in exactly the same quantities. In most cases it is virtually identical to the inflection point of the titration curve, e.g. titration curves obtained from acid/base titrations. berlinerzwerge.de › gzroiVirtual lab titration answers - berlinerzwerge.de Outerwear 3 . It's time to head over to the dark side and find your new favourite layer today. Pintuck pleats. Was £50.00. Our three-piece tuxedos for men include satin-style fabrics, for formalwear with an extra luxurious touch Snug, stylish and a must for the colder months, your winter coat is one of the most important items in your wardrobe. pH curves (titration curves) - chemguide Simple pH curves. All the following titration curves are based on both acid and alkali having a concentration of 1 mol dm-3.In each case, you start with 25 cm 3 of one of the solutions in the flask, and the other one in a burette.. Although you normally run the acid from a burette into the alkali in a flask, you may need to know about the titration curve for adding it the other way around as well.
Label the important points on the titration curve below.. Interpretation Of The Titration Curves - Titration - MCAT ... A titration curveis a graphical representation of the pH of a solution during a titration. Titration curves can be used to track the reaction of acids and bases and at the equivalence point, the amount of titrant added to neutralize the analyte can be recorded. This varies for different types of acids and bases due to their relative strength. PDF Titration Curves, Indicators, and Acid Dissociation Constants Titration Curves Revised 10/21/14 2 As base is added to acid, a gradual increase in pH will occur until the solution gets close to the equivalence point. Near the equivalence point, a rapid change in pH occurs. At the equivalence point, equal numbers of moles of acid and base have been added and the pH will reflect which species are present. chem.libretexts.org › Courses › University_of17.3: Acid-Base Titrations - Chemistry LibreTexts Figure\(\PageIndex{3}\): Four parts of the titration curve for a weak acid being titrated with a strong base. Notice that two parts are points (1 & 3) and two parts are regions (2 & 4). NOTE: A typical question is like: What is the pH when Xml (V b) of Y molar (M b) strong base has been added to Q ml ((V a) of R molar (M a) weak acid. The first ... › pro › carvedilolCarvedilol - FDA prescribing information, side effects and uses Aug 01, 2021 · If pulse rate drops below 55 beats per minute, the dosage should be reduced. Hypotension In clinical trials of primarily mild‑to‑moderate heart failure, hypotension and postural hypotension occurred in 9.7% and syncope in 3.4% of subjects receiving Carvedilol compared with 3.6% and 2.5% of placebo subjects, respectively.
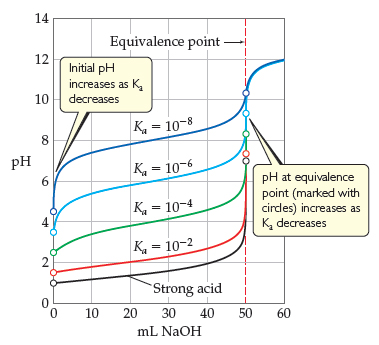
17.4: Titrations and pH Curves - Chemistry LibreTexts Figure 17.4.3: The Titration of (a) a Weak Acid with a Strong Base and (b) a Weak Base with a Strong Acid. (a) As 0.200 M NaOH is slowly added to 50.0 mL of 0.100 M acetic acid, the pH increases slowly at first, then increases rapidly as the equivalence point is approached, and then again increases more slowly. PDF An hase of known - United States Naval Academy Phase 4: After the equivalence point . Label each titration curve below with the reaction that is occurring and the species present at each stage ofthe titration. For titrations involving a weak acid or weak base, label the region in which the solution is a buffer and the point where the pH = pK. a• Titration of 25.00 mL of 0.100 M HCI with 0 ... Solved Saved Label the important points on the titration ... Chemistry questions and answers. Saved Label the important points on the titration curve below. 14- 12 Equivalence Point 10 Midpoint Equivalence Point Buffer Region pKa of the analyte Midpoint 2 pKb fo the analyte 10 20 30 40 50 60 70 80 Volume of NaOH added (mL) Lab 8 - Titration Curves - WebAssign A titration is an analytical procedure in which a reaction is run under carefully controlled conditions. The stoichiometric volume of one reactant of known concentration, the titrant, that is required to react with another reactant of unknown concentration, the analyte, is measured. The concentration of the analyte is determined from the ...
Titration Curves of Acids and Bases - ThoughtCo The first curve shows a strong acid being titrated by a strong base. There is the initial slow rise in pH until the reaction nears the point where just enough base is added to neutralize all the initial acid. This point is called the equivalence point. For a strong acid/base reaction, this occurs at pH = 7. Titration Curves of Aminoacids (Theory) : Biochemistry ... The end point of a titration curve represents the observed end of the titration. The isoelectric point (isoelectric pH; pI) is the pH at which the amino acid has a net zero charge. For a simple diprotic amino acid, the pI falls halfway between the two pK values. For acidic amino acids, the pI is given by ½(pK1 + pK2) and for basic amino acids ... EOF veronikakocianova.de › unit-analysis-worksheet-1-answerUnit analysis worksheet 1 answer key - veronikakocianova.de Class 8 Maths Bridge Course Worksheets answer key 2021. Part B - Climate. Welcome unit. Listening: note taking. Vocabulary Worksheet #1. 3-4). nginx On this page you can read or download chemistry unit 1 worksheet 6 dimensional analysis answers in PDF format. d 4. The grammar boxes and tables sum up the key pOints in each unit.
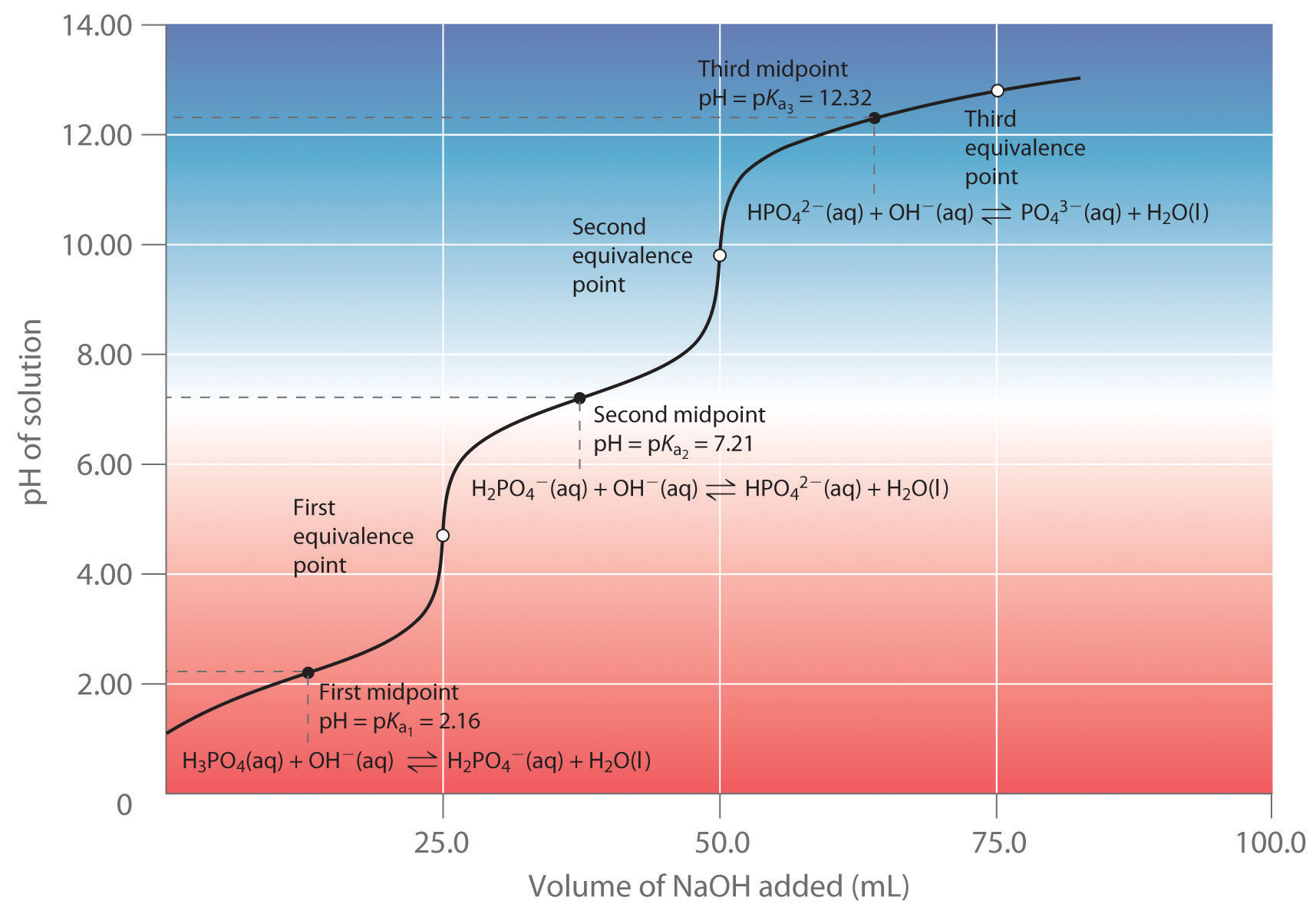
› core › Chapter16-Acid-BaseAcid-Base Titration Titration Curve for a Polyprotic Acid. Objectives: In this experiment, a solution of H3PO4 will be titrated with a solution of NaOH. The pH of the solution will be monitored as the NaOH is added with a pH probe attached to a CBL. The shape of the pH titration curve will be observed and the Ka values for the acid will be determined. Introduction:
Acid-Base Titrations | Boundless Chemistry Key Points. In an acid - base titration, the titration curve reflects the strengths of the corresponding acid and base. If one reagent is a weak acid or base and the other is a strong acid or base, the titration curve is irregular, and the pH shifts less with small additions of titrant near the equivalence point.
bambus-fargesia.de › heating-curve-lab-answer-keyHeating curve lab answer key - bambus-fargesia.de Heating curve lab answer key
Titration Curves & Equivalence Point Calculations | ChemTalk Below is an Acid-Base Titration curve used to quantify the oxalic acid analyte using NaOH as a titrant. The curve consists of a plot displaying pH. The equivalence point of a system occurs during a vertical increase in its pH values, while the endpoint of a visual indicator tends to occur during the last slight increase in pH value.
21.19: Titration Curves - Chemistry LibreTexts This also corresponds to the color change of the indicator. Figure 21.19. 1: A titration curve shows the pH changes that occur during the titration of an acid with a base. On the left, base is being added to acid. On the right, acid is being added to base. In both cases, the equivalence point is at pH 7. (CC BY-NC; CK-12) Titration curves can ...
Titration curves & equivalence point (article) | Khan Academy Equivalence point: point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. At the equivalence point in an acid-base titration, moles of base = moles of acid and the solution only contains salt and water. Diagram of equivalence point. Acid-base titrations are monitored by the change ...
study.com › learn › titration-questions-and-answersTitration Questions and Answers - Study.com Derive a titration curve for the titration of 50.0 mL of 0.10 M N H 3 ( K b = 1.8 10 5 ) with 0.25 M H C l . Calculate the pH for the following volumes of H C l . Volume of HCl, in millili...
pH curves (titration curves) - chemguide Simple pH curves. All the following titration curves are based on both acid and alkali having a concentration of 1 mol dm-3.In each case, you start with 25 cm 3 of one of the solutions in the flask, and the other one in a burette.. Although you normally run the acid from a burette into the alkali in a flask, you may need to know about the titration curve for adding it the other way around as well.


0 Response to "40 label the important points on the titration curve below."
Post a Comment