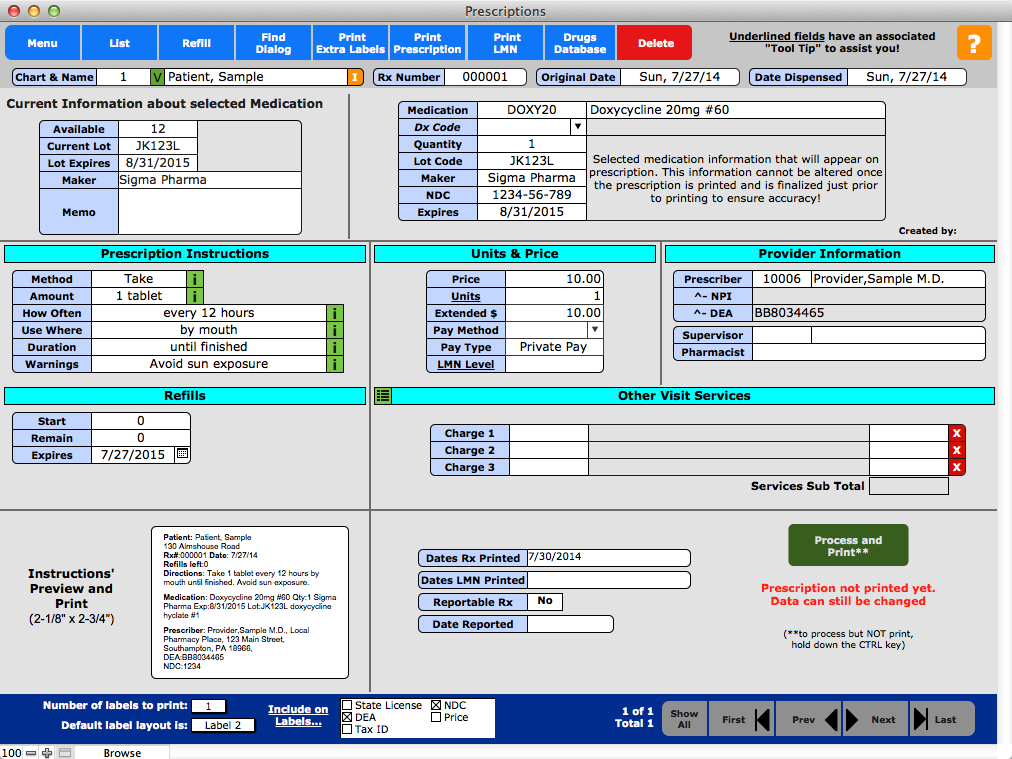
41 what is required on a prescription label
Introduction to the New Prescription Drug Labeling by the FDA A prescription drug product label (also known as a professional label, package insert, direction circular, and package circular) is a compilation of information about a product written by the... Medical prescription - Wikipedia A prescription, often abbreviated ℞ or Rx, is a formal communication from a physician or other registered health-care professional to a pharmacist, authorizing them to dispense a specific prescription drug for a specific patient. Historically, it was a physician's instruction to an apothecary listing the materials to be compounded into a treatment—the symbol ℞ (a capital …
RCW 18.64.246: Prescriptions—Labels—Cover or cap to meet ... (1) to every box, bottle, jar, tube or other container of a prescription which is dispensed there shall be fixed a label bearing the name and address of the dispensing pharmacy, the prescription number, the name of the prescriber, the prescriber's directions, the name and strength of the medication, the name of the patient, the date, and the …

What is required on a prescription label
Prescription Label Template - Fill Out and Sign Printable ... All you need is smooth internet connection and a device to work on. Follow the step-by-step instructions below to eSign your blank prescription label template: Select the document you want to sign and click Upload. Choose My Signature. Decide on what kind of eSignature to create. There are three variants; a typed, drawn or uploaded signature. PDF Labeling Requirements - California The California Patient Medication Safety Act (Chapter 470, Statutes 2007) requires the Board of Pharmacy to promulgate regulations on or before January 1, 2011, that require a standardized, patient-centered prescription drug container label for all prescription ·drugs dispensed to patients in California. Label - Wikipedia A label (as distinct from signage) is a piece of paper, plastic film, cloth, metal, or other material affixed to a container or product, on which is written or printed information or symbols about the product or item. Information printed directly on a container or article can also be considered labelling.. Labels have many uses, including promotion and providing information on a …
What is required on a prescription label. The Pharmaceutics and Compounding Laboratory The Pharmaceutics and Compounding Laboratory 6800.3400 PRESCRIPTION LABELING. - Minnesota Veterinary prescription drug label. ... When the veterinary drug is in the manufacturer's original package and the information that is required on the label includes the drug or drugs, strength of the drug or drugs, directions for use, withdrawal time for food-producing animals, and cautionary statements, a label will be required on each ... PDF Issuing a Valid Prescription - Ohio Prescribers may electronically transmit prescriptions directly to the pharmacy. Except for a manual signature, the systems must be able to transmit all the required information as required on a written prescription. It is the responsibility of the vendor and prescriber to ensure that all prescriptions include the required information listed in Guidelines for Prescription Labeling | American Foundation ... Provide "duplicate labels" (prescription and auxiliary) printed in a minimum of 18-point type on paper stock. If pictograms are used, these should also be provided in "large print" format and high contrast (saturated black on white background).
FDA Issues New Prescription Label Requirements | The ... A prescription drug label must include certain information. FDA prescription labeling requirements must be clearly printed with: Pharmacy information Doctor information Instructions Physical description of the drug Federal caution statement Dates Pharmacy prescription number Number of pills Number of times the drug can be reordered Pharmacist FAQs - NCBOP A. The following information must be on every prescription label: 1. Name and address of the dispensing pharmacy. 2. Serial number of the prescription. 3. Date of the prescription. 4. Name of the prescriber. 5. Name of the patient. 6. Name and strength of the drug. 7. Prescriptions- Label Flashcards - Quizlet Here is a list of all the things that need to go on a prescription label for a non-control: 1. name of pharmacy 2. address of pharmacy 3. telephone number of pharmacy 4. number of the prescription 5. date the prescription is filled 6. name of patient**** 7. name of prescriber 8. initials of the pharmacist dispensing the Rx Quick Guide: How to Make a Fake Prescription Label ... You will be prompted to select the label width, height, quantity, application, adhesive, and color. All these play a vital role in making a good label. Fill in the information. This will require information like the name of the pharmacy, name of the medicine, patient's details, prescription details, and more.
What Is a Pesticide? — Beyond Pesticides According to the Environmental Protection Agency (EPA), the government body that regulates pesticides in the U.S., a pesticide is any substance or mixture of substances intended for preventing, destroying, repelling or mitigating any pest. Though often misunderstood to refer only to insecticides ... DOC NEW PRESCRIPTION REQUIREMENTS - Texas A sample prescription is included with this Q&A. Note: Although a prescription format is no longer required, prescriptions issued by APNs and PAs must still conform to the requirements specified in Section 157.056 of the Medical Practice Act. Prescription Label Information, Translations, and Sample ... Prescription Label Information, Translations, and Sample Labels. Translations of Pill Directions ; Patient-Centered Prescription Drug Container Label Samples; Prescription Drugs: Labeling Requirements - Report to the Legislature; Statutory Requirements (4076.5) and Regulation Requirements (1707.5) 2022 LAWBOOK FOR PHARMACY The Pharmacy Law ... - California Caution Label Required for Drug Containing an Opioid . 4077. Dispensing Dange rous Drug in Incorrectly Labeled Container . 6 . 4078. False or Misleading Label on Prescription 4079. Availability of a Lower Retail Price for a Covered Drug . Article 5. …
Prescription Drug Labeling Medication Errors: A Big Deal ... 20.7.2006 · Evidence suggests that specific content and format of prescription drug labels facilitate communication with and comprehension by patients. Efforts to improve the labels should be guided by such evidence, although an additional study assessing the influence of label design on medication-taking behavior and health outcomes is needed.
Prescription Drug Labels (9-29-2014) | Georgia Drugs and ... (1) before an out-patient prescription drug is released from the dispensing area, the prescription drug shall bear a label containing the name and address of the pharmacy, a prescription number, the name of the prescriber, the name of the patient, directions for taking the medication, the date of the filling or refilling of the prescription, the …
Pharmacist FAQs - NCBOP A. The following information must be on every prescription label: 1. Name and address of the dispensing pharmacy. 2. Serial number of the prescription. 3. Date of the prescription. 4. Name of the prescriber. 5. Name of the patient. 6. Name and strength of the drug. 7.
Prescription Drug Labeling Resources | FDA The Prescribing Information is written for the healthcare practitioner and must: Contain a summary of essential scientific information needed for safe and effective use of the human prescription...
What Information Should Be on Drug Labels? FDA Labeling ... Prescription vs. over-the-counter drug label requirements The regulations are different for prescription and over-the-counter drugs . Here is an easy-to-understand walkthrough of the guidelines.
What is required on prescription label? The minimum requirements on prescription labels for most drugs are as follows: name and address of dispenser, prescription serial number, date of prescription or filling, name of prescriber, name of patient, directions for use, and cautionary statements. The number assigned by the manufacturer. What must be shown on a prescription?
Packaging and Labeling of Pharmaceutical Products Obtained ... In the United States, the legal requirements for a prescription label are set by federal law and state statutes [2,3]. At the federal level, the required items of information for the prescription product label can be found in Section 503 (b) (2) of the Federal Food, Drug, and Cosmetic Act (Table 1) .
Illinois (IL) - Prescription label requirements Pharmacy name Pharmacy address Date and initials of person authorized to dispense Name of patient Serial number of prescription Prescriber's last name Directions for use Quantity Dosage Name …
FDA Regulation of Animal Drugs | FDA 10.8.2021 · Prescription (Rx) products can be ... The following information must be included on the label of the dispensed prescription animal ... of each active ingredient as well as the information required ...
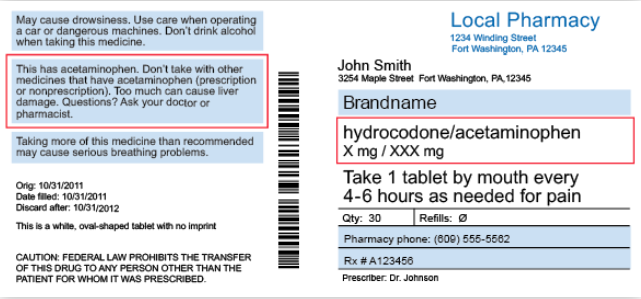
Education Understanding Prescription Medication Labels ... Understanding Prescription Medication Labels Prescription Medication Label Overview Whenever you are prescribed a medication, you must be sure that you understand the key sections of the medication's label in order to ensure your safety. The label on your prescription bottle contains information from your doctor and your pharmacy about using your medication correctly. Use the…
New York (NY) | Prescription label requirements In addition, such drug shall bear a label containing the proprietary or brand name of the drug and, if applicable, the strength of the contents, unless the person issuing the prescription explicitly states on the prescription, in his own handwriting, that the name of the drug and the strength thereof should not appear on the label.
A Guide To Veterinary Prescription Label Requirements ... What Is Required On A Veterinary Prescription Label As shown in the above example, the actual container must include the following information: The name of the veterinary practice, its address, and contact information The veterinarian's name, the patient's name and species, and the client's last name
Prescription Label Flashcards | Quizlet Whenever you are prescribed a medication, you must be sure that you understand the key sections of the medication's label in order to ensure your safety. The label on your prescription bottle contains information from your doctor and your pharmacy about using your medication correctly.
Section 12-505 - Labeling requirements for prescription ... Labeling requirements for prescription medicines. (a) Label required.- Except for a drug or device dispensed to an inpatient in a hospital or related institution, each container of a drug or device dispensed shall be labeled in accordance with this section.
What information is required on my prescription ... In order for a prescription to be valid it must include: Patient Name (full name, no initials) Date prescription was written Drug Name (Brand name, Generic name if available) Drug Strength (How many mg, mEq, grams, etc.) Dosage Form (Tablets, Capsules, Ointment, Creams, Spray, etc.)
Label - Wikipedia A label (as distinct from signage) is a piece of paper, plastic film, cloth, metal, or other material affixed to a container or product, on which is written or printed information or symbols about the product or item. Information printed directly on a container or article can also be considered labelling.. Labels have many uses, including promotion and providing information on a …
PDF Labeling Requirements - California The California Patient Medication Safety Act (Chapter 470, Statutes 2007) requires the Board of Pharmacy to promulgate regulations on or before January 1, 2011, that require a standardized, patient-centered prescription drug container label for all prescription ·drugs dispensed to patients in California.
Prescription Label Template - Fill Out and Sign Printable ... All you need is smooth internet connection and a device to work on. Follow the step-by-step instructions below to eSign your blank prescription label template: Select the document you want to sign and click Upload. Choose My Signature. Decide on what kind of eSignature to create. There are three variants; a typed, drawn or uploaded signature.










0 Response to "41 what is required on a prescription label"
Post a Comment