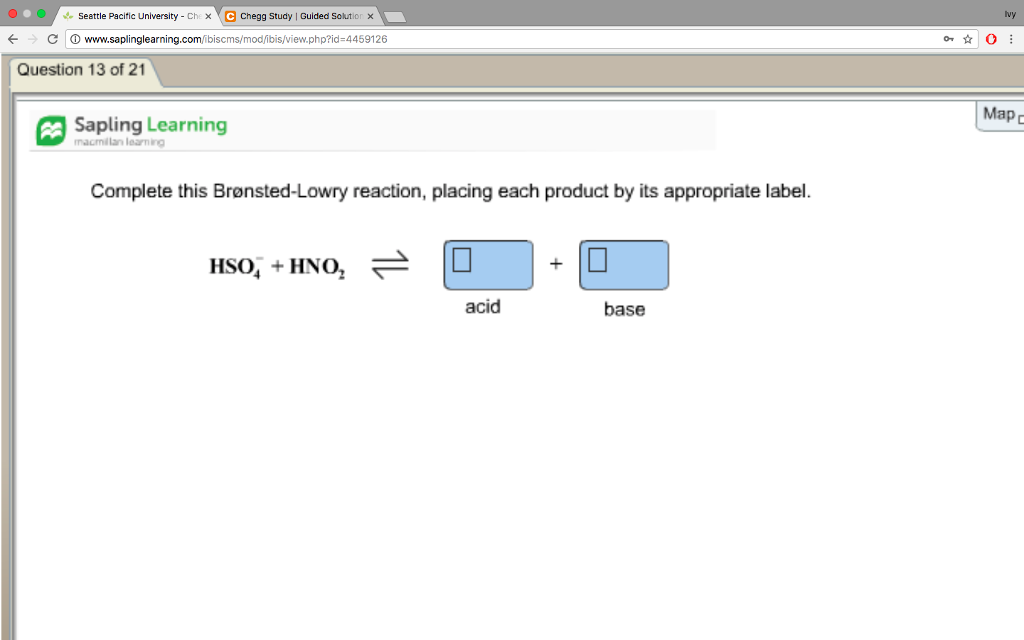
44 complete this brønsted-lowry reaction, placing each product by its appropriate label.
Bronsted-Lowry Base: Bronsted-Lowry Reactions & Examples complete this Bronsted- Lowry reaction, placing each product by its appropriate label. HCN3- + CN; Question 2 1) What is the conjugate base of HCO_3^-? Express your answer as a chemical formula. How many moles of o2 are consumed if 20 moles of so2 are produced in the given question, the partial pressure is calculated by using the ideal gas equation, that is, pv = nrt here, p is pressure, v is volume = 25 l, n is number of moles = 0.0104 moles, r is gas constant = -0.0821 l.atm / mol.k t is temperature = 273 k p = nrt / v p = (0.0104 mol × 0.0821 l.atm / mol.k × 273 k) / 25 l = 0.0093 atm
Conjugate Acid Questions and Answers - Study.com Complete the following reaction. nitric acid + sodium sulfide . ... Complete this Bronsted-Lowry reaction, placing each product by its appropriate label. ... placing each product by its ...
Complete this brønsted-lowry reaction, placing each product by its appropriate label.
Solved Complete this Brønsted-Lowry reaction, placing each | Chegg.com Complete this Brønsted-Lowry reaction, placing each product by its appropriate label. HSO4^-+BrO^-<---> First identify whether BrO- is an acid or a base. This will help you determine whether the amphoteric substance HSO4- is acting as an acid or a base in this reaction. SO4^2- + HOBr and HSO4- + HOBr are not correct Expert Answer Question : Complete this Bronsted-Lowry reaction, placing each product ... Complete this Bronsted-Lowry reaction, placing each product by its appropriate label. HSO4^- + HBrO Question: Complete this Bronsted-Lowry reaction, placing each product by its appropriate label. HSO4^- + HBrO This problem has been solved! See the answer Show transcribed image text Expert Answer 86% (7 ratings) 👋 Solved Solutions 📚 - EssayShark starting at $13.40 per page 📚 The first-order reaction SO2Cl2→ SO2 + Cl2 is 10% complete in 80. min. How long would it take for the reaction to be 95% complete? A. 1.8 min B. 104 min C. 530 min D. 2300 min E. 990 min When the re… “Please discuss about””cosmeceuticals””. Whether or not you think cosmeceuticals should be reclassified as drugs or cosmetics, or ...
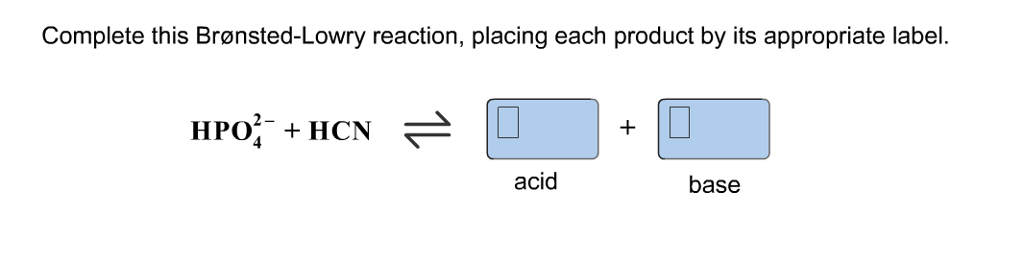
Complete this brønsted-lowry reaction, placing each product by its appropriate label.. Harris Quantitative Chemical Analysis 8th edition - Academia.edu Enter the email address you signed up with and we'll email you a reset link. Complete this brønsted-lowry reaction. placing each product by its ... Complete this brønsted-lowry reaction. placing each product by its appropriate label. Answer According to Bronsted-Lowry reaction, an acid is the one which can donate H+ ions & a base is the one which absorbs H+ ions. HPO 42- + BrO - HBrO + PO 43- acid base Maybe you like Balance the following redox reactions occurring in acidic aqueous solution? Chemistry vocabulary and answer Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn. Answers: 1. Answer. Chemistry, 22.06.2019 03:30 ... acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction? Answers: 1. Answer. You know the right answer? Based on their locations on the periodic table, which best compares the ... Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn. Answers: 1. Answer. Chemistry, 22.06.2019 11:00. Imagine that twenty i.u.'s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7 ...
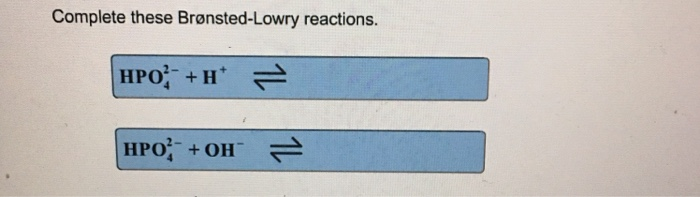
Solved Complete this Brønsted-Lowry reaction, placing each | Chegg.com Chemistry questions and answers Complete this Brønsted-Lowry reaction, placing each product by its appropriate label. HPO BrO acid base Question:Complete this Brønsted-Lowry reaction, placing each product by its appropriate label. HPO BrO acid base This problem has been solved! See the answerSee the answerSee the answerdone loading Complete these Bronsted-Lowry reactions. HPO^2-_4 - Transtutors Complete this Bronsted-Lowry reaction, placing each product by its appropriate label. HPO^2-_4 + BrO^- doubleheadarrow HBrO + H_2PO^-_4 acid base Posted 2 years ago. Q: Complete these Bronsted-Lowry reactions. H2PO4- + H+ H2PO4- + OH- Posted 2 years ago ... Complete this Bronsted-Lowry reaction, placing each product by it's ... Complete these bronsted lowry reactions HS^- + H^+ ---> HS^- + OH^- ----> Chemistry In aqueous solution, hypobromite ion, BrO-, reacts to produce bromate ion, BrO3 -, and bromide ion, Br-, according to the following chemical equation. 3BrO- (ag) → BrO3 - (ag)+2Br- (ag) A plot of 1/ [BrO-] vs. time is linear and the Chemistry Complete the Bronsted- Lowry reaction, placing each product by its ... Complete the Bronsted- Lowry reaction, placing each product by its appropriate label. HCO3^-+HNO2 (double arrows) need a spot for acid and for base. 34,418 results Chem Complete the Bronsted lowry reactions HPO42-+H+ HPO42-+OH- CHEMISTRY Complete these bronsted lowry reactions HS^- + H^+ ---> HS^- + OH^- ----> CHEMISTRY
use the bronsted-lowry model to label the acid-base pairs in the following use the bronsted-lowry model to label the acid-base pairs in the following equation for the ionization of water: H20(L) + H20(L) H30+(AQ) + OH-(AQ). EXAPLAIN 57,224 results Chemistry For the reaction CH3COOH- CH3COO + H+, which of the following statements is true? CH3COO- is an Arrhenius base. Join My Meetingid-9384026088password 1234 Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn Solved Complete this Brønsted-Lowry reaction, placing each | Chegg.com This problem has been solved! See the answer Complete this Brønsted-Lowry reaction, placing each product by its appropriate label. HSO 4- +BrO - ACID? + Base? Expert Answer HSO4- +BrO- <--- … View the full answer Previous question Next question Complete this Bronsted-Lowry reaction, placing each product by it's ... A Bronsted-Lowry acid is a proton donor. True or False Chemistry For the reaction 2CO2 (g) + 5H2 (g)C2H2 (g) + 4H2O (g) H° = 46.5 kJ and S° = -124.8 J/K The standard free energy change for the reaction of 1.75 moles of CO2 (g) at 328 K, 1 atm would be ___ kJ This reaction is (reactant, product) ____ favored under standard Chemistry
Solved Complete this Brønsted-Lowry reaction, placing each | Chegg.com See the answer Complete this Brønsted-Lowry reaction, placing each product by its appropriate label. HCO3- + F- <=> ___+___ acid + base Expert Answer 100% (4 ratings) HCO3 (-) + F (-) = CO3 (2-) + HF her … View the full answer Previous question Next question
Identify the products formed in this Brønsted-Lowry reaction. - Jiskha Complete this Bronsted-Lowry reaction, placing each product by it's appropriate label. HSO4- + BrO- ____ (ACID) + ____ (BASE) chemistry HCN (aq) + SO4-2 (aq) HSO4- (aq) + CN - (aq) a. What is the Bronsted - Lowry acid in this equation? b. What is the Bronsted - Lowry base in this equation? c. What is the conjugate acid in this equation? d.
(PDF) Quantitative Chemical Analysis 7E Daniel C. Harris ... Quantitative Chemical Analysis 7E Daniel C. Harris
Complete this Brønsted-Lowry reaction, placing each product by its ... 1 Answer The Bronsted-Lowry definition of acid and base says that the acid donates H+, and the base accepts it. In this equation, the HSO4- ion actually accepts the H+ from HNO2, to form H2SO4 + NO2-. So the HSO4- acts as the base, while the HNO2 acts as the acid.
0 Response to "44 complete this brønsted-lowry reaction, placing each product by its appropriate label."
Post a Comment