44 label the tonicity for each solution
Hypertonic, Hypotonic, Isotonic: Guide to Fluid Balance in the Body I put this chart here to help you visualize why the IV solution is hypertonic, hypotonic or isotonic. The number of particles in the D5W is 50, and this is a hypotonic solution. Whereas D5 + Ringer's is a hypertonic solution and it has 361 particles. Patient scenarios. Your patient has had nausea, vomiting, and diarrhea for 4 days. PDF Key Cell Membrane and Tonicity Worksheet - Copley A SOLUTION is a combination of solute and solvent. The process by which water diffuses across a membrane called OSMOSIS Part II - Look at the solutions illustrated above and fill in the blanks. 1. Solution B is HYPERTONIC to Solution A. This is because Solution B has a greater concentration of SOLUTES in it than does Solution A.
Chapter 5 Osmolarity & Tonicity Questions and Study Guide - Quizlet 3) tonicity of a solution describes the volume change of a cell at equilibrium 4) determine tonicity by comparing non penetrating solute concentrations in the cell and the solution. net water movement is into the compartment with the higher concentration of non penetrating solutes 5) hyposmotic solutions are always hypotonic concentration

Label the tonicity for each solution
Isotonic, Hypertonic, and Hypotonic Solutions The solution outside the cell is what we are referring to when we talk about isotonic, hypertonic, or hypotonic. The solution may be pure water or the solution may be water with a solute dissolved in it, or any such solution. For the below examples, we will use a cell that has a NaCL concentration of 0.9%. So the water concentration inside of ... Tonicity - an overview | ScienceDirect Topics Tonicity is one of the factors that affects drug absorption. Shrinkage of epithelial cells is a common feature in presence of hypertonic solutions. On the other hand, hypertonic saline solutions also cause the inhibition of ciliary activity. The tonicity of the administered form of the drug significantly affects the nasal mucosa; so generally ... Osmolarity and Osmolality - an overview | ScienceDirect Topics The tonicity of the solution is an important clinical issue. Complete understanding of the tonicity concept requires differentiation of two terms, osmolality and osmolarity. Osmolality is the number of dissolved particles per kilogram of solution and is expressed as mOsm/kg of solution.
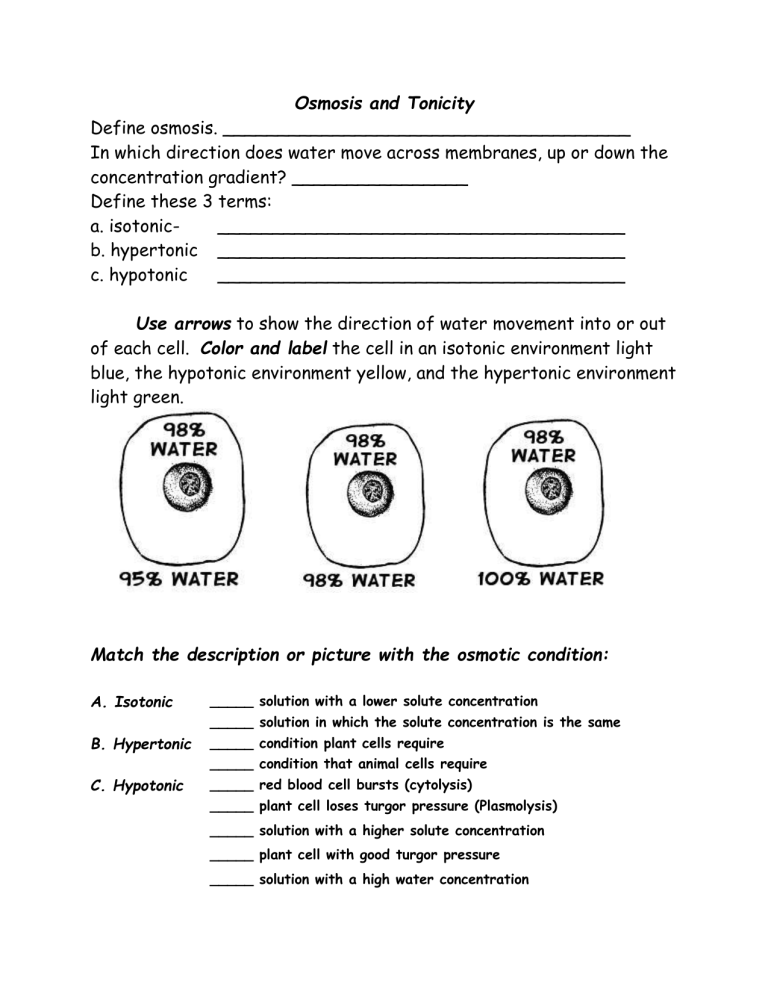
Label the tonicity for each solution. 2 Label the tonicity for each solution isotonic hypotonic or hypertonic ... Complete the table below by placing a in the correct column (s) next to the description. Hypertonic Hypotonic Isotonic 1. Causes an animal cell to burst/lyse. 2. Causes a plant cell to become wilted. 3. When the solute concentration is lower outside the cell than inside the cell. 4. Does not change the shape of volume of a cell. 5. Week 6 Cell Membrane & Tonicity Worksheet.pdf - Course Hero Color and Label the transport proteins red and the substance being moved blue. Label and color the carrier proteins red and the ions green. TONICITY AND OSMOSIS Part I - Fill in the blanks. A Solvent is a fluid in which a substance is dissolved. A Solute is a substance dissolved in a solvent. A Solution is a combination of solute and solvent. Tonicity | Hypotonic, Hyertonic & Isotonic Solutions - iBiologia Tonicity is that the capability of a solution because of which water will interchange into or out of a cell by the method of diffusion is phenomena is named Osmotic Pressure. Tonicity of any solution is associated with its solutions Osmolarity. Osmolarity is the overall concentration of all solutes solution within the solution. DOC Name______________________ Date_________ Period_________ to show the direction of water movement into or out of each cell. Color and label. the cell in an isotonic environment light blue, the hypotonic environment yellow, and the hypertonic environment light green. Match the description or picture with the osmotic condition:
Cell membrane and tonicity worksheet Key - StuDocu __ A __ plant cell with good turgor pressure. __ C___ solution with a high water concentration. Label the tonicity for each solution (isotonic, hypotonic, ... Answered: Below is a diagram of two solutions… | bartleby Label the tonicity of each solution relative to each other (and show your calculations) and draw an arrow indicating the direction of water movement. Dıici=2.07g/mL 15% v/v LİCI 4 M LİCI Question thumb_up 100% Transcribed Image Text: Below is a diagram of two solutions separated by a water-permeable membrane. DOC Cell Transport Review Worksheet - Cabarrus County Schools Color and Label. the transport proteins red and the substance being moved blue. Complete the table by checking the correct column for each statement: Statement Isotonic solution Hypotonic solution Hypertonic solution Causes a cell to swell Doesn't change the shape of a cell Causes osmosis Causes a cell to shrink Isotonic, Hypotonic & Hypertonic IV Fluid Solution Tonic: concentration of a solution The cell has a low amount of solute extracellularly and it wants to shift inside the cell to get everything back to normal via osmosis. This will cause CELL SWELLING which can cause the cell to burst or lyses. Hypotonic solutions 0.45% Saline (1/2 NS) 0.225% Saline (1/4 NS) 0.33% saline (1/3 NS)
PDF Lab #5: Osmosis, Tonicity, and Concentration. - ANBA First, determine the number of moles of solute used to make the solution by dividing the mass of the solute by its molecular weight. 1) Moles Solute = Mass (g) Molecular weight (g/mole) Then divide the number of moles solute by the volume of the solution in liters. 2) Molarity (M) = Moles Solute Solution Volume (L) ADRIAMYCIN (DOXOrubicin HCl) for Injection May 16, 2012 · Each 2 mg/mL, 10 mL (20 mg) vial contains 20 mg Doxorubicin Hydrochloride, USP; Sodium Chloride 0.9% (to adjust tonicity) and Water for Injection q.s.; pH adjusted to 3 using Hydrochloric Acid. Each 2 mg/mL, 25 mL (50 mg) vial contains 50 mg Doxorubicin Hydrochloride, USP; Sodium Chloride 0.9% (to adjust tonicity) and Balanced Multielectrolyte Solution versus Saline in ... Jan 18, 2022 · Abstract Background Whether the use of balanced multielectrolyte solution (BMES) in preference to 0.9% sodium chloride solution (saline) in critically ill patients reduces the risk of acute kidney ... Cell Membrane & Tonicity Worksheet _____ plant cell with good turgor pressure. _____ solution with a high water concentration. Label the tonicity for each solution (isotonic, hypotonic, ...
Label the tonicity for each of the following solulions ... the three terms isotonic solution Cremation and Humala sis usually applied to red blood cells. Not always, but in this case will look at it.
Tonicity Definition, Types, Examples Temporary hydrogen bonds are formed between water molecules and other solutes due to the different electrical poles of each watermolecule. This allows water to be distributed and moved into new areas. ... Water's tonicity is enhanced by adding solutes, but it is impossible to label the solution using one specific term of tonicity without ...
DOC Name______________________ Date_________ Period_________ to show the direction of water movement into or out of each cell. Color and label. the cell in an isotonic environment light blue, the hypotonic environment yellow, and the hypertonic environment light green. ... ____ plant cell with good turgor pressure _____ solution with a high water concentration Label the tonicity for each solution ...
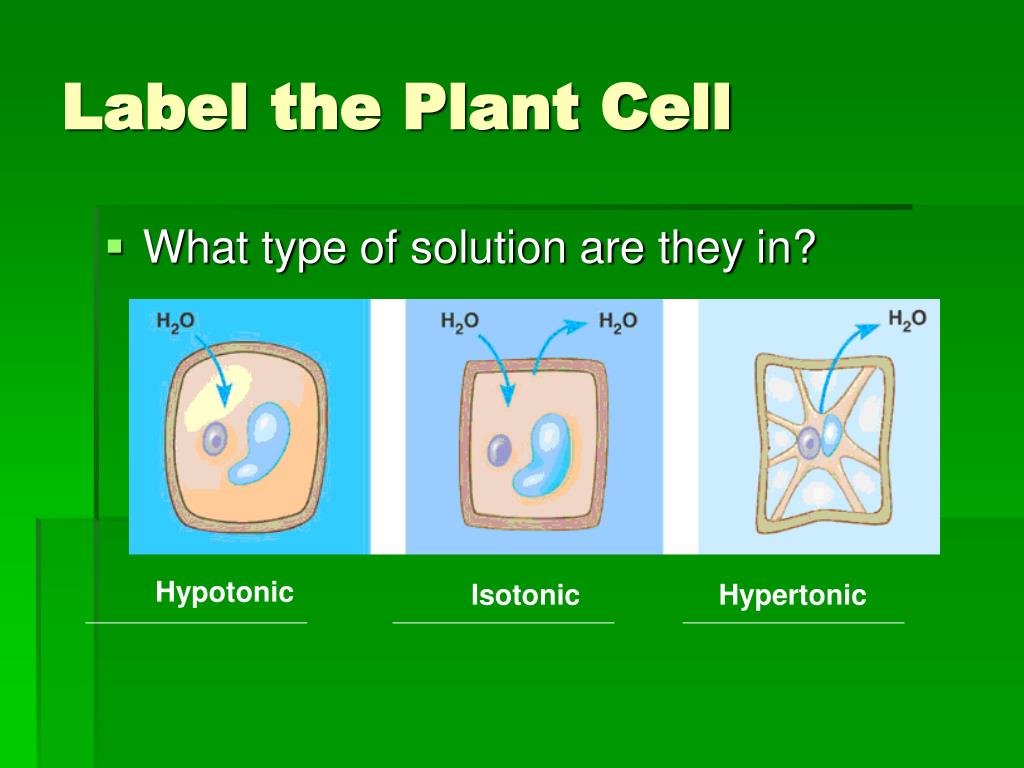
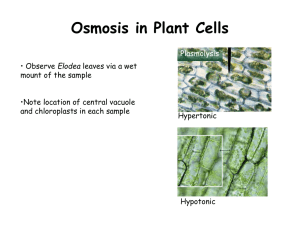
PDF KEY Cellular Transport Worksheet - Lloyd M. Clarke Label the tonicity for each solution (isotonic, hypotonic, or hypertonic): Pay close attention to the arrows!!! _hypotonic_ _isotonic_ _hypertonic_ _hypotonic_ _isotonic_ _hypertonic_ Examine the pictures on the bottom of the left side of this page. What [if anything] is different about the plant and animal cells in each of these states? ...
PDF 1. SKETCH AND LABEL a phospholipid coloring the heads red and ... - BIOLOGY _____ solution with a higher solute concentration _____ plant cell with good turgor pressure _____ solution with a high water concentration 9. Label the tonicity for each solution (isotonic, hypotonic, or hypertonic): 10. Transport Requiring Energy What type of transport is represented by the following picture?
Tonicity Diagram - schematron.org Particles in solution are generally free to . Hypertonic, isotonic, and hypotonic solutions and their effect on cells. Formally, osmosis is the net movement of water across a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration. Three terms—hypotonic, isotonic, and.
Solved: Label each solution as isotonic, hypotonic, or hypertonic ... 87QP Label each solution as isotonic, hypotonic, or hypertonic in comparison to 0.9% (m/V) NaCl (0.15 M NaCl). 0.15 M CaCl 2 Step-by-step solution Step 1 of 3 is an electrolyte which dissociates in water to form two mol of particles: Chapter 6, Problem 87QP is solved. View this answer View a sample solution Step 2 of 3 Step 3 of 3 Back to top
Reference ID: 3677449 - Food and Drug Administration Oral Solution 20%: Each 15 mL of solution contains 3.0 g of potassium chloride, USP and the following inactive ingredients: citric acid anhydrous, FD&C Yellow #6, glycerin, methylparaben, natural/artificial orange flavor, propylene glycol, propylparaben, purified water, sodium citrate dihydrate, sucralose.



0 Response to "44 label the tonicity for each solution"
Post a Comment