40 draw the product of the lewis acid-base reaction. label the electrophile and nucleophile.
Answered: Draw the products of each Lewis… | bartleby Draw the products of each Lewis acid-base reaction. Label the electrophile and nucleophile. CH3 CH3 + H2SO4 CH3 CH3 + H20 Question Transcribed Image Text:Draw the products of each Lewis acid-base reaction. and nucleophile. CH3 CH3 + H2SO4 CH3 CH3 + H20 Expert Solution Want to see the full answer? Check out a sample Q&A here See Solution star_border Draw the product of the Lewis acid-base reaction. Label the ... - OneClass Get the detailed answer: Draw the product of the Lewis acid-base reaction. Label the electrophile and
Draw the product of the Lewis acid-base reaction and label the ... We have to give the product of the reaction and label the electrophile and the nucleophile. According to the theory of Lewis acids and bases, an...

Draw the product of the lewis acid-base reaction. label the electrophile and nucleophile.
Chapter 1: Acid-Base Reactions - Michigan State University Lewis Acids and Bases, Electrophiles and Nucleophiles. As we have seen, any reaction in which a proton (H +) is transferred from one molecule to another can be considered as a Lewis acid-base reaction, but now it is time to broaden the scope of Lewis acid-base reactions. The structural requirement for a Lewis base is essentially the same as ... Draw the products of each Lewis acid-base reaction. Label the ... This is the answer to chapter to problem number 65 from the Smith Organic Chemistry textbook. Uh, in this problem gives us five reactions and asks us to label the nuclear file and Elektra file and each reaction on then to draw the products. So I'm gonna go through and label nuclear files and electric files first on and then go back through and ... (Get Answer) - -Sort the following bases in decreasing order of ... -Sort the following bases in decreasing order of basicity. F-, -OH, -NH2 , -CH3 -Label the reagents for these reactions as Lewis acids (electrophile) or as the Lewis base (nucleophile). Use curved arrows to show the movement of the electron pairs in the reactions. -Classify the following species in order: a) Increasing acidity:
Draw the product of the lewis acid-base reaction. label the electrophile and nucleophile.. (PDF) Study Guide and Solutions Manual to Accompany T.W. 30-11-2014 · Study Guide and Solutions Manual to Accompany T.W. Graham Solomons / Craig B. Fryhle / Scott A. Snyder / Jon Antilla [Solved] Please see an attachment for details | Course Hero Use the pka table in Appendix A to decide if the equilibrium favors the starting materials or products. OH O=0 a. + -OCH2CH3 d. + NaHCO, CF3 OH b. + NaCI e. H-C C-H + Lit CH,CH3 CH,CH2 OH C. (CH3)3COH + H2SO4 f. CH,NH2 + H2SOA . Question 5: Draw the products of each Lewis acid-base reaction. Label the electrophile and nucleophile. CHa a. Nucleophiles and Electrophiles — Organic Chemistry Tutor What is a Nucleophile. A nucleophile is a "nucleus loving" species if we look at the word itself and translate its Greek roots. The nucleophiles are typically negatively charged or have at least one electron pair they can easily share to make a new chemical bond. For instance, the CH 3 O - and CH 3 NH 2 are a couple of examples of common ... Lewis Acid: Definition, Theory & Examples - Study.com Draw the product formed when the Lewis acid reacts with the Lewis base (CH_3)_2NH . For Co3+, write an equation that shows how the cation acts as an acid. Express your answer as a chemical equation.
Draw the products of each reaction, and label the nucleophile and ... VIDEO ANSWER: Whenever I start any sort of reaction in organic chemistry, I asked myself where all potential nuclear files are. So where is any access electron… Solved Be sure to answer all parts. Draw the product of the | Chegg.com Expert Answer 100% (3 ratings) Positively charged and electron deficient species are called electrophile … View the full answer Transcribed image text: Be sure to answer all parts. Draw the product of the Lewis acid-base reaction. Label the electrophile and nucleophile. F -F CH3CH,OH + BF3 H₂CHEC edit structure ... nucleophile lectrophile PDF Nucleophiles and Electrophiles - University of Texas at Austin such as a neutral B atom. It might be a good idea to review Lewis acids and Lewis bases in Section 4.7 of the book before proceeding. The new species held together by the new coordinate bond is commonly referred to as a Lewis acid-Lewis base complex. 2. When the Lewis acid is a proton source such as HCl, it is referred to as a Brønsted- Solved Be sure to answer all parts. Draw the product of the - Chegg Question: Be sure to answer all parts. Draw the product of the Lewis acid-base reaction. Label the electrophile and nucleophile. CHCH,OH + BF3 draw structure (select) (select) This problem has been solved! See the answer Show transcribed image text Expert Answer 100% (2 ratings)
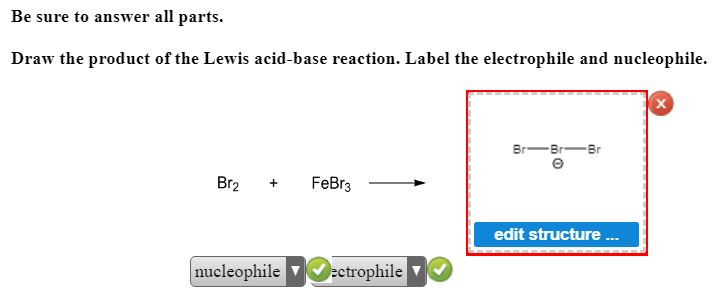
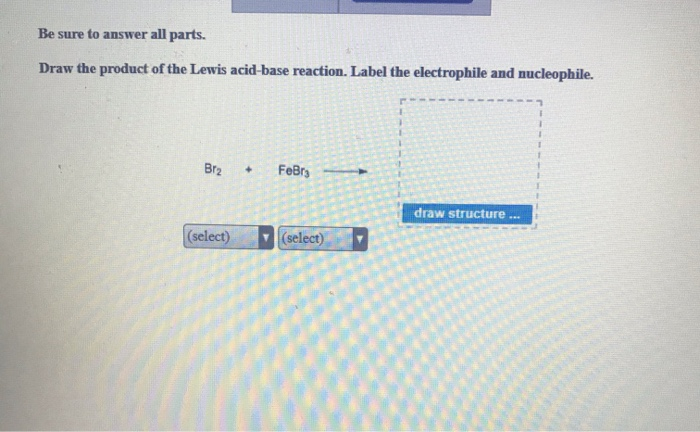
Stanford University UNK the , . of and in " a to was is ) ( for as on by he with 's that at from his it an were are which this also be has or : had first one their its new after but who not they have Draw the product of the Lewis acid-base reaction. Label the ... Answer to: Draw the product of the Lewis acid-base reaction. Label the electrophile and the nucleophile. Br_2 + FeBr_2 rightarrow ? Inorganic Chemistry by Miessler ~ 5th Edition - Academia.edu This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. Draw the product of the Lewis acid-base reaction. Label the ... Answer to: Draw the product of the Lewis acid-base reaction. Label the electrophile and nucleophile. By signing up, you'll get thousands of...
SOLVED:Label the Lewis acid and Lewis base in each reaction. Use curved ... label Louis Acid and Louis based in each pair and add curved arrow notation, otherwise known as a mechanism to get from the starting materials to the products. So for this first compound, we start with steel minus BCL free in order to form be seal four minus.
Nucleophilic Aromatic Substitution: Introduction and Mechanism 20-08-2018 · 4. The Effect Of The Leaving Group. One of the most eye-opening aspects of nucleophilic aromatic substitution is noting that fluorine is often used as a leaving group. This is seen in Sanger’s reagent for sequencing peptides, to take one example (more on that below). After all, given the stern tones we instructors use in Org 1 on this subject, the words …
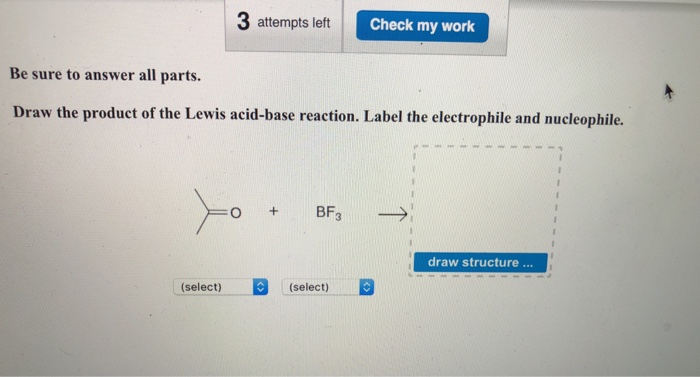
Answered: • Question 5: Draw the products of each… | bartleby Label the electrophile and nucleophile. CH₂ a. CH₂CH₂OH + BF₁ C=O+BF₁- - 0. Br₂+ FeBr - CH₂ b. CH₂SCH, + AICI, + H₂O - C. Expert Solution Want to see the full answer? Check out a sample Q&A here See Solution star_border Students who've seen this question also like: Chemistry Chemical Foundations. 1RQ expand_more Want to see this answer and more?
Draw the product of the Lewis acid-base reaction. Label the ... - OneClass State the product or products and write a detailed mechanism for the following reaction. In addition, label the Lewis acid, Lewis base, nucleophile and electrophile Weekly leaderboard
OneClass: Be sure to answer all parts. Draw the product of the reaction ... Draw the product of the Lewis acid-base reaction. Label the electrophile and nucleophile. Br_2 + FeBr_5 rightarrow
(Get Answer) - -Sort the following bases in decreasing order of ... -Sort the following bases in decreasing order of basicity. F-, -OH, -NH2 , -CH3 -Label the reagents for these reactions as Lewis acids (electrophile) or as the Lewis base (nucleophile). Use curved arrows to show the movement of the electron pairs in the reactions. -Classify the following species in order: a) Increasing acidity:


0 Response to "40 draw the product of the lewis acid-base reaction. label the electrophile and nucleophile."
Post a Comment