45 label each asymmetric carbon in the compound below as r or s.
[Solved] For each structure, (i) Star (*) any asymmetric carbon atoms ... Kindly get the solution for the five parts step by step Step-by-step explanation Image transcriptions solution : (1) asymmetric carbon atoms - For each structure, (ii) Label each asymmetric carbon as (R) or (S). (iii) Draw in any internal mirror planes of symmetry. (iv) Label the structure as chiral or achiral. (v) Label any meso compounds. b) Finding Asymmetrical Carbons in R and S Isomers - Yeah Chemistry In the following compound label the asymmetric carbon (s) present as either R or S: a) CH3CH (CH3)C (Br) (CH3)C (OH) (CH3)CH3 b) CH (Br) (Cl)C (CH3) (OH)CH2CH3 I was looking at some of the other problems, and I am stumped on how the instructor determined the asymmetric carbons.
study.com › academy › lessonChiral vs. Achiral: Definition & Examples - Study.com Nov 23, 2021 · Label each stereogenic center as R or S. In the following compounds, find the number of stereocenters and chiral centers, and classify them as chiral or achiral. Build a model of CH_2BrCl.

Label each asymmetric carbon in the compound below as r or s.
HOMEWORK 2 (1).docx - NAME: _ CHEM 2423 HOMEWORK 2 1) How ... - Course Hero 3 ) Assign the proper configurational label , R or S , to each chiral carbon in the molecule below . 2 ) Given that glucose has a specific rotation of + 52.8 ° . Predict the concentration of a glucose aqueous solution contained in a 10 cm long polarimetry tube if a rotation of + 15.8 ° was observed . Label The Following Compounds As Having R Or S Configuration Around The ... 8) label each asymmetric carbon in the compound below as r or s. Stereocenters are labeled r or s. 2) for compound 2 the configuration is r. Assigning 'r and s' configuration to chiral centers: Label the following compounds as having r or s configuration around the stereocenter(s). This problem has been solved! Answered: Label the pair of compounds below as:… | bartleby Solution for Label the pair of compounds below as: CH3 CH3 H. H. and H. H. H. stereoisomers O. identical, but differing in conformation O constitutional isomers…
Label each asymmetric carbon in the compound below as r or s.. What is an Asymmetric Carbon? - Study.com Definition of Asymmetric Carbon An asymmetric carbon atom is defined as a carbon within an organic compound that contains four different atoms or groups of atoms (substituents) bonded to it. As an... Answer the following questions A Each of the following compounds ... Locate each of these chirality centers and identify the configuration of each one. B) Captopril is used to treat high blood pressure and congestive heart failure. Label the chiral centers as R or S. C) Label each asymmetric carbon in the compound below as R or D) Identify the compounds that are not chiral. S. Answered: Q3 Configurations (Wade 5-26) For each… | bartleby Q3 Configurations (Wade 5-26) For each structure, 1. star any asymmetric carbons, 2. label each asymmetric carbon as R or S, 3. draw any internal mirror planes of symmetry, 4. label each structure as chiral or achiral, 5. label any meso structures. H. Solved Label each asymmetric carbon in the compound below as | Chegg.com Label each asymmetric carbon in the compound below as R or S Note: not all targets will be used. COH R CHyH ; Question: Label each asymmetric carbon in the compound below as R or S Note: not all targets will be used. COH R CHyH
Solved enantiomer HO 8) Label each asymmetric carbon in the | Chegg.com OH CH3 9) Label each asymmetric carbon in the compound below as R or S OH CH3 CH3 OH 10) Label each assymetric carbon in the compound below as R or S. Cl 11) Draw the structure of (2R,3S)-2,3-dichloropentane. Take particul dimensional stereochemical detail properly. 12) Draw the This problem has been solved! See the answer SOLVED:24) Draw the Fischer projection of (S)-2 hydroxybutanoic acid ... So this compound starts with a six carbon chain 123456 Hence the name hexen. And we will have chlorine groups are carbons two and four. ... diastereomers the_same compound? CHa "H HaC CHzCH3 CHzCH3 28) Label each asymmetric carbon in the molecule below as having the R or $ configuration. HOzC H3c CHzCH3 CH3 Question 4 draw the most stable conformation of cis 1 - Course Hero Question 4 Draw the most stable conformation of cis-1-t-butyl-4-methylcyclohexane. Answer: Question 5 How many enantiomers are there of the molecule shown below? Answer: 1. Answer : 4 Question 6 Circle each chiral molecule among those shown below. Answer: Question 7 Label each asymmetric carbon in the molecule below as having the RorSconfiguration. chem.libretexts.org › Courses › Athabasca_University26.1 Structures of Amino Acids - Chemistry LibreTexts Sep 22, 2020 · Why is cysteine the only L amino acid with an R configuration at the alpha carbon? Q26.1.2. Isoleucine has two stereogenic centers. (a) Draw a Fischer projection of isoleucine. (b) Draw a Fischer projection of an isoleucine diastereomer, and label each stereocenter as R or S.
PRACTICE QUESTIONS FOR CH. 5 PART I H NH 2 - Academia.edu CH3 H3C OH HO 8) Label each asymmetric carbon in the compound below as R or S. OH CH3 9) Label each asymmetric carbon in the compound below as R or S. OH H CH3 H CH3 OH 10) Label each assymetric carbon in the compound below as R or S. Cl 11) Draw the structure of (2R,3S)-2,3-dichloropentane. Solved Label each asymmetric carbon in the compound below as - Chegg Label each asymmetric carbon in the compound below as R or S. Question : Label each asymmetric carbon in the compound below as R or S. This problem has been solved! OneClass: Label each asymmetric carbon in the compound below as R or S ... This is counterclockwise (S), however since the hydrogen is coming towards us it should be flipped to R. the second asymmetric carbon is attached to CH3, CH2(right) , CH2(left) and CH (bottom) so priority order: CH3 1st, CH2 left 2nd and CH2 right 3rd and lastly the CH that is attached to O. So I got clockwise so R CI 2 Br Hac H2C Answer +20 Watch pubchem.ncbi.nlm.nih.gov › compound › 4_4_-Diphenyl4,4'-Diphenylmethane diisocyanate | C15H10N2O2 - PubChem Diphenylmethane-4,4-diisocyanate is a light yellow colored solid.It is not soluble in water.It may be toxic by ingestion, inhalation, or skin absorption. If in a solution it may or may not burn depending on the nature of the material and/or the solvent.
en.wikipedia.org › wiki › TireTire - Wikipedia Wet traction—Wet traction is the tire's traction, or grip, under wet conditions. Wet traction is improved by the tread design's ability to channel water out of the tire footprint and reduce hydroplaning. However, tires with a circular cross-section, such as those found on racing bicycles, when properly inflated have a sufficiently small ...
› doi › 10Scaffold hopping by net photochemical carbon deletion of ... Apr 28, 2022 · Accordingly, a pressing challenge and increasing recent area of focus for modern organic synthesis is the development of transformations that can address the molecular skeleton with precision and enable direct scaffold hops between distinct core substructures within a given class of compounds.
Answered: For each structure, star (*) any… | bartleby label each asymmetric carbon as (R) or (S). draw any internal mirror planes of symmetry. label the structure as chiral or achiral. label any meso structures. *refer to the photo below Transcribed Image Text: For each structure, star (*) any asymmetric carbon atoms. 2. label each asymmetric carbon as (R) or (S). 3.
What the **** OChem???? Flashcards - Quizlet Given a DG° of 0.8 kJ/mol at 25°C for the equilibrium shown below, calculate the percentage of the axial conformer at 25°C. [R = 8.314 J/K∙ mol] C If the equilibrium constant (Keq) of a reaction is 0.5 then which of the following that must be true? A) The reaction will have an early transition state. B) Gibbs free energy (G) is negative.
en.wikipedia.org › wiki › GrapheneGraphene - Wikipedia Graphene (/ ˈ ɡ r æ f iː n /) is an allotrope of carbon consisting of a single layer of atoms arranged in a two-dimensional honeycomb lattice nanostructure. The name is derived from "graphite" and the suffix -ene, reflecting the fact that the graphite allotrope of carbon contains numerous double bonds.
OneClass: How many asymmetric carbons are present in the compound below ... For each molecule below, first indicate whether it is chiral or achiral. Then identify any asymmetric carbons, if they exist, in each molecule, and assign their stereochemistry as (R) or (S). (Each molecule may have 0, 1, or more than 1 asymmetric carbon!) Finally, if a molecule is a mesa compound, label it as such. D. F Cl Cl CI Cl D F D H
ch5_stereo1.docx - PRACTICE QUESTIONS FOR CH. 5 PART I 1 ... - Course Hero View ch5_stereo1.docx from CHEMISTRY 131 at San Francisco State University. PRACTICE QUESTIONS FOR CH. 5 PART I 1) Is the molecule shown below chiral or achiral? OH OH 2) Is the molecule shown below
333467903 276543468 Organic Chemistry Wade Test Bank - StuDocu 44) Captopril is used to treat high blood pressure and congestive heart failure. Label the chiral centers as R or S. Answer: Diff: 3 Section: 5. 45) Label each asymmetric carbon in the compound below as R or S. Answer: Diff: 1 Section: 5. 46) Draw the structure of ( S)-1-bromo-1-chloropropane. Take particular care to indicate three- dimensional ...

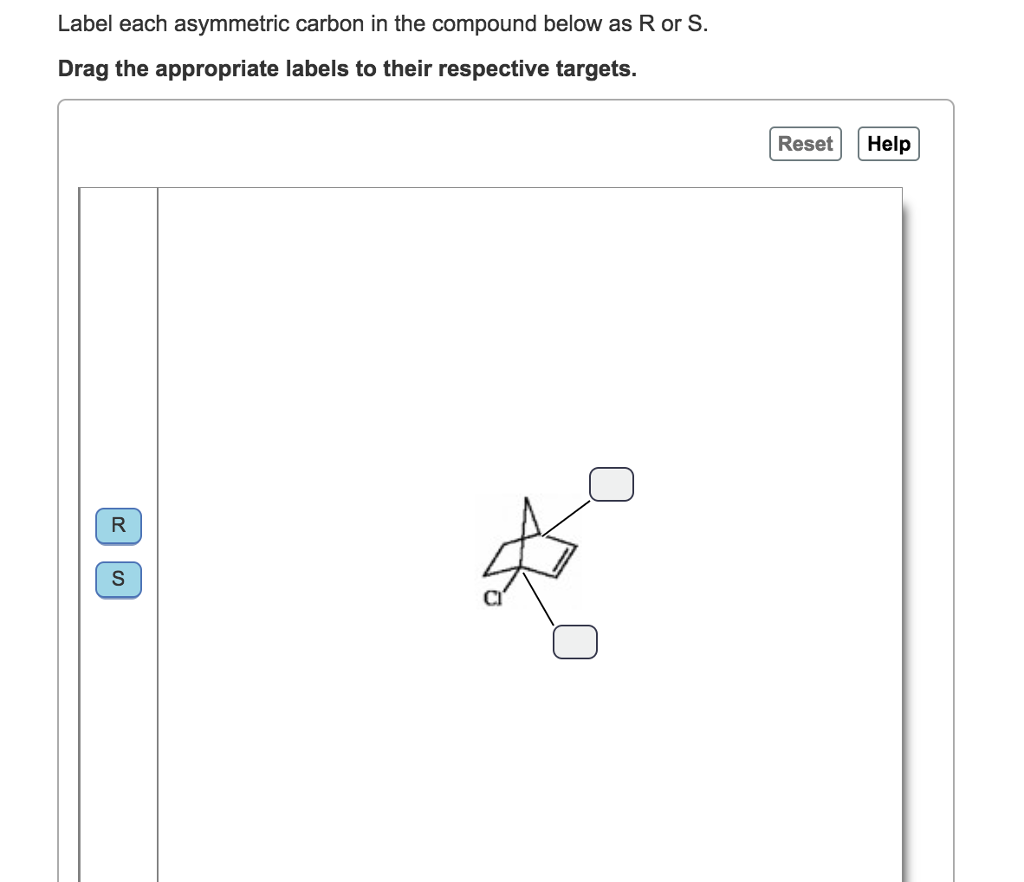



0 Response to "45 label each asymmetric carbon in the compound below as r or s."
Post a Comment