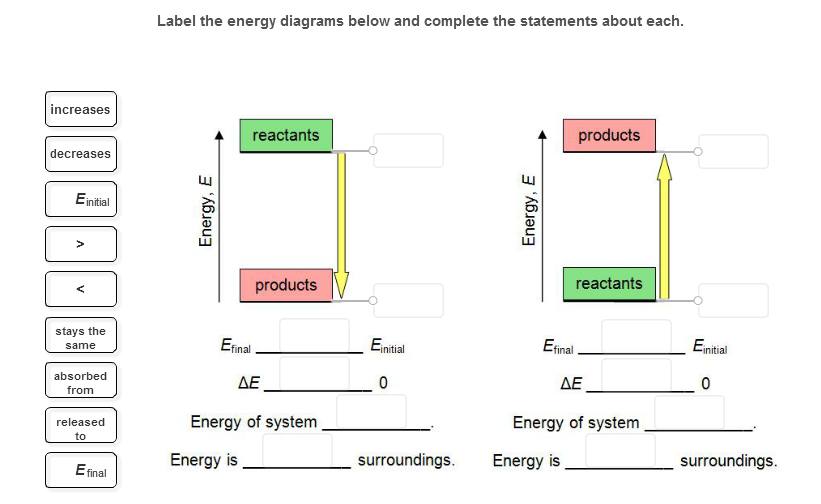
44 label the reactants and products on the enthalpy diagrams for each process.
Enthalpy - Chemical energy - Higher Chemistry Revision - BBC This means that the enthalpy change is the difference in energy between the products and the reactants. The enthalpy change takes the form of heat given out or absorbed. The heat energy given out... ENERGY PROFILES FOR SIMPLE REACTIONS - chemguide This diagram shows that, overall, the reaction is exothermic. The products have a lower energy than the reactants, and so energy is released when the reaction happens. It also shows that the molecules have to possess enough energy (called activation energy) to get the reactants over what we think of as the "activation energy barrier".
Solved Label the reactants and products on the enthalpy | Chegg.com Question: Label the reactants and products on the enthalpy diagrams for each process. Endothermic Exothermic Enthalpy Enthalpy Answer Bank products reactants This problem has been solved! See the answer Show transcribed image text Expert Answer 100% (14 ratings) For an exothermic just keep in mind that it gives off heat at the …
Label the reactants and products on the enthalpy diagrams for each process.
Draw an enthalpy diagram for a general exothermic reaction ... - Quizlet Find step-by-step Chemistry solutions and your answer to the following textbook question: Draw an enthalpy diagram for a general exothermic reaction; label the axis, reactants, products, and $\Delta \mathrm { H }$ with its sign.. Label the total change in enthalpy δh and activation The change in enthalpy is positive because the heat is added to the system. It will become negative if the heat is removed from the system. ENERGY BALANCE: Energy In = Energy Out E IN = E OUT Heat Added (Ea) + Enthalpy In (H IN) = Enthalpy Out (H OUT) Heat Added (Ea) = Enthalpy Out (H OUT) - Enthalpy In (H IN) Ea = ΔH Endothermic vs. exothermic reactions (article) | Khan Academy Exothermic reactions: Heat is released. 1) Combustion: The burning of carbon-containing compounds uses oxygen, from air, and produces carbon dioxide, water, and lots of heat. For example, combustion of methane () can be represented as follows: 2) Rain: Condensation of water vapor into rain releasing energy in the form of heat is an example of ...
Label the reactants and products on the enthalpy diagrams for each process.. Solved Label the reactants and products on the enthalpy Question: Label the reactants and products on the enthalpy diagrams for each process. Endothermic Exothermic Enthalpy Enthalpy Answer Bank reactants products ... Answered: Draw an energy pathway diagram for the… | bartleby Label the axes, reactants, products, enthalpy change, activation energy, and activated complex on your diagram. C (9) + O2g) CO2 (9) + 393.5 kJ Question 1 Transcribed Image Text: please help Draw an energy pathway diagram for the following reaction. How to Draw & Label Enthalpy Diagrams - Study.com This enthalpy diagram has starting products, ending products, delta H, and activation energy labeled There are two different types of energy changes in reactions, endothermic and exothermic.... SOLVED:Label the reactants and products 0 the enthalpy diagrams for ... Hello here we have to label reactions and the products on the internal P. Tech er diagram for his reaction. Let's start with an atomic reaction. So in an ender thermic reaction the the age yes positive. That's why products have higher entropy and reactors will be lower in. Meanwhile, for the exit ceramic reaction it's the opposite.
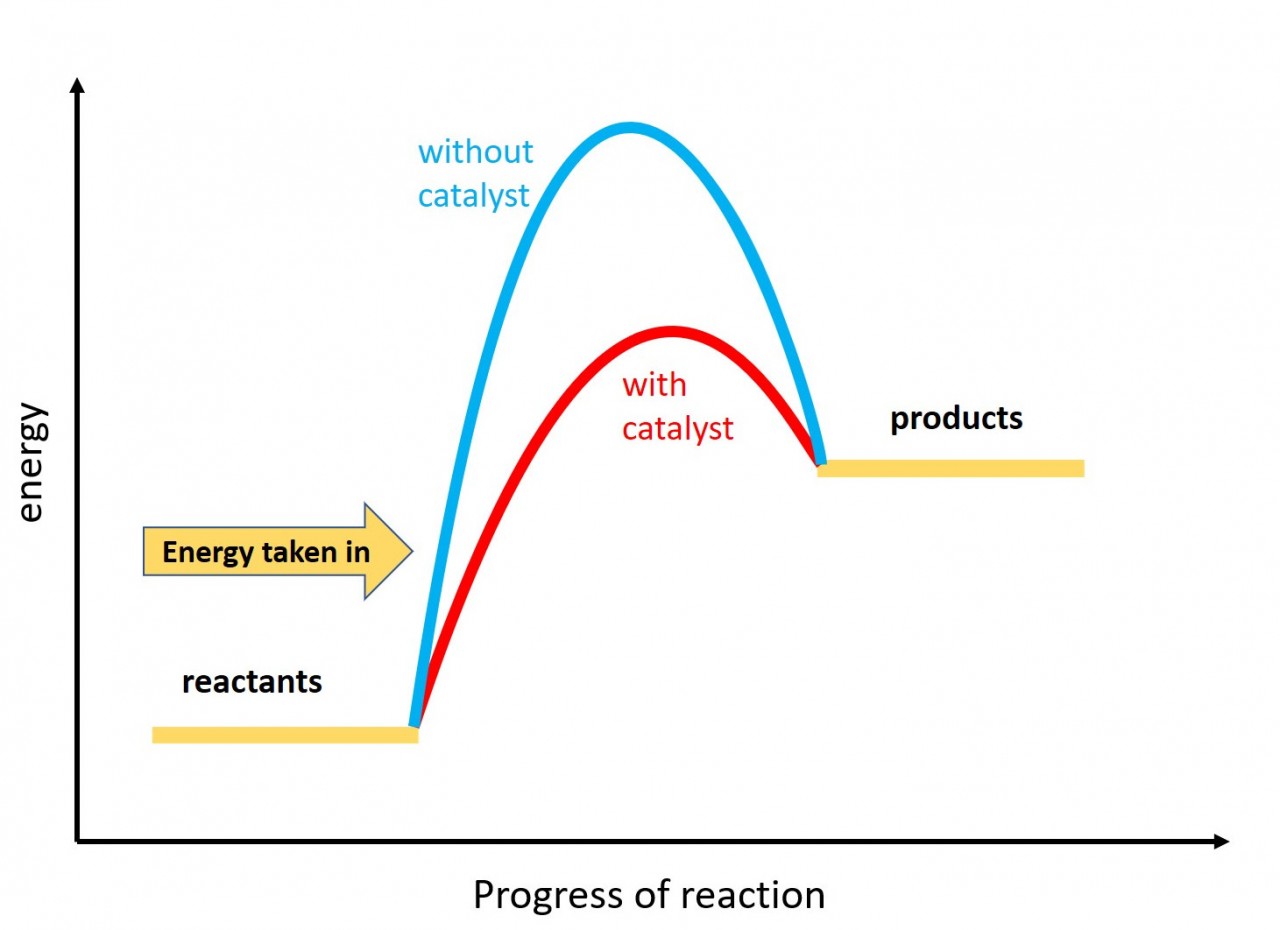
PDF Topic 5.1 Exothermic and Endothermic Reactions Heat and Temperature a) Draw a diagram of the energy profile for this reaction. Label the diagram. b) State whether the reaction is endothermic or exothermic. c) Calculate the energy difference between the reactants and the products. d) Deduce the sign of the enthalpy change. e) Identify with a reason, which is more stable, the reactants of products. 8. (N04/S/2) How does the energy level diagram show this reaction is exothermic? Label ΔH as positive or negative. Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases. Energy Diagrams of Reactions | Fiveable ΔH is the difference between the potential energy of the products and the potential energy of the reactants. Simply do: (PEproducts) - (PEreactants) (20 kJ) - (40 kJ) = -20 kJ In other words, 20 kJ of energy is released during this reaction. (d) What is the activation energy? PDF Activation Energy - Oklahoma State University-Stillwater B. Sketch the reaction profile (reaction energy diagram) illustrated in the simulation. Label the sketch using the following terms: potential energy, reactants, products, reaction progress, activation energy, enthalpy, and collision energy. Determine values for the activation energy and enthalpy and include them in your diagram
Solved Label the reactants and products on the enthalpy Question: Label the reactants and products on the enthalpy diagrams for each process. Endothermic Exothermic Enthalpy Enthalpy · This problem has been solved! PDF Representing a Reaction with a Potential Energy Diagram The reactants will be shown as the first level portion, and the products will be the second level portion of the diagram. The activation energy in the forward direction, E a(fwd), is the difference between the reactant energy and the transition state at the peak of the diagram. The activation energy for the reverse reaction, E Label the reactants and products on the enthalpy diagrams for each process. Q: At 552.3 k, the rate constant for the thermal decomposition of f so2cl2 is 1.02k2*10^-6. If the activation energy is 210kj/mol determine the rate constant at 700k. Posted 26 days ago. Q: CHM 703 Electro-analytical Techniques 1a. Explain the principle of anodic stripping voltammetry. Why is stripping the most sensitive polarographic technique? Answered: Draw an enthalpy diagram for a general… | bartleby Solution for Draw an enthalpy diagram for a general exothermic reaction; label the axis, reactants, products, and ΔH with its sign. close. Start your trial now! First week only $4.99! arrow ... The process of losing electrons is called oxidation and the process of losing electrons is called re ...
Energy Profiles (Energy Diagrams) Chemistry Tutorial - AUS-e-TUTE enthalpy of reactants = enthalpy of products + energy released H (N 2 (g) and H 2 (g)) = H (NH 3 (g)) + 92.4 kJ mol -1 We know the enthalpy change for the reaction: ΔH = -92.4 kJ mol -1 . (Remember the minus sign (-) tells us energy is released, energy is a product of the reaction, the reaction is exothermic.)
How to draw the potential energy diagram for this reaction? 2. Identify the sign of the enthalpy change, ΔH, along with its value. The decrease in chemical potential energy should be the same as the amount of thermal energy released. That is. Ereactants − Eproducts = 2219.9lkJ⋅ mol−1. However, since. ΔH = Eproducts −Ereactants , ΔH = −2219.9lkJ⋅ mol−1. Note that by convention, the ...
Label the x and y axes the reactants and the products - Course Hero Label the x- and y-axes, the reactants, and the products on the diagram. Label the total change in enthalpy (ΔH) and activation energy (Ea) on your diagram. Make sure correct units are included.
Endothermic Exothermic Reactions - Jack Westin A negative enthalpy change for a reaction indicates exothermic process, while a positive enthalpy change corresponds to endothermic process. In order to calculate the standard enthalpy of reaction, we need to look up the standard enthalpies of formation for each of the reactants and products involved in the reaction.
What potential energy diagram shows? - Brainly.com The difference between the chemical potential energy of the products and the reactants is the enthalpy of the reaction: ΔH rxn = ΔH products - ΔH reactants. The labels that correspond to each part of the diagram are explained next. 2) Reactants: This is the substances at the start, so they appear on the left bottom side of the diagram.
Label thc rcactants and products 0n the enthalpy diagrams for each ... SOLVED:Label thc rcactants and products 0n the enthalpy diagrams for each process; Endothermic Exothermic j f Hello, everybody In this question, it's asking us to draw on entropy diagram for an exo thermic reaction. So the first thing I'm gonna do is label my axes. So the X axis right here is going to be time.



0 Response to "44 label the reactants and products on the enthalpy diagrams for each process."
Post a Comment