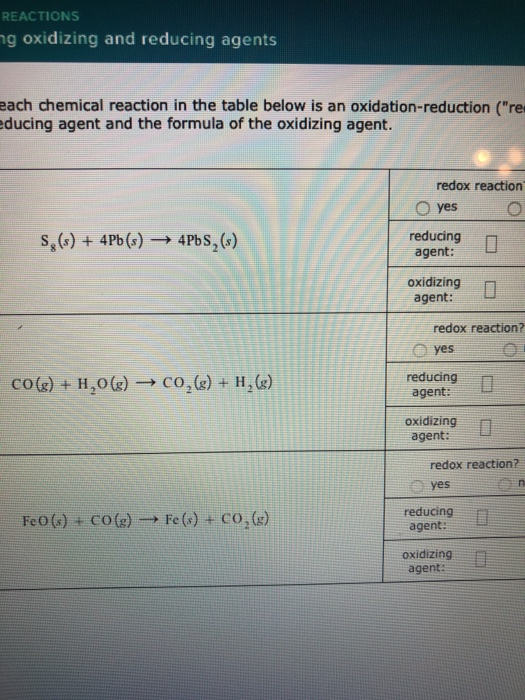
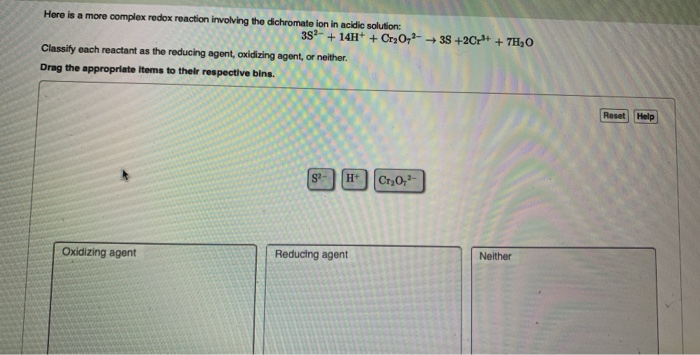
39 label each reactant as the reducing agent, oxidizing agent, or neither.
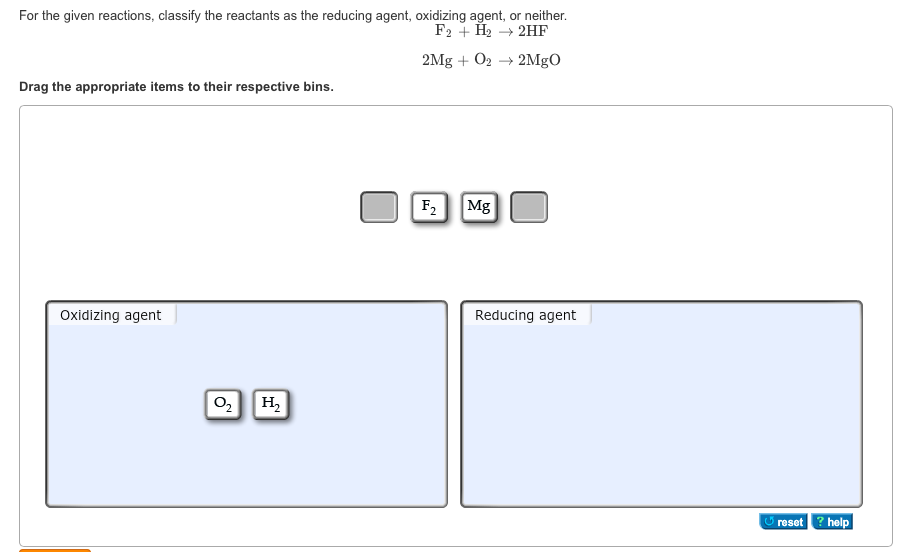
GEN CHEM Final Flashcards | Quizlet Study with Quizlet and memorize flashcards containing terms like For the given reactions, classify the reactants as the reducing agent, oxidizing agent, or neither. F2 + H2 → 2HF 2Mg + O2 → 2MgO, Classify each reactant as the reducing agent, oxidizing agent, or neither. 3S2− + 14H+ + Cr2O72− → 3S +2Cr3+ + 7H2O, Indicate whether each statement is true or false. (a) Separate ... Learn About Redox Problems With an Example - ThoughtCo To summarize, -2 electrons per oxygen atom, +3 electrons for each iron atom. 2 Al: The oxidation number of a free element is always zero. Al 2 O 3: Using the same rules for Fe 2 O 3, we can see there are -2 electrons for each oxygen atom and +3 electrons for each aluminum atom. 2 Fe: Again, the oxidation number of a free element is always zero.
3NO2− + 8H+ + Cr2O72− → 3NO3− +2Cr3+ + 4H2O Classify each reactant as ... answered • expert verified 3NO2− + 8H+ + Cr2O72− → 3NO3− +2Cr3+ + 4H2O Classify each reactant as the reducing agent, oxidizing agent, or neither. Advertisement milliezenip4oel8 is waiting for your help. Add your answer and earn points. superman1987 According to this reaction: 3NO2^- + 8 H^+ + Cr2O7^-2 → 3NO3^- + 2Cr^+3 + 4 H2O
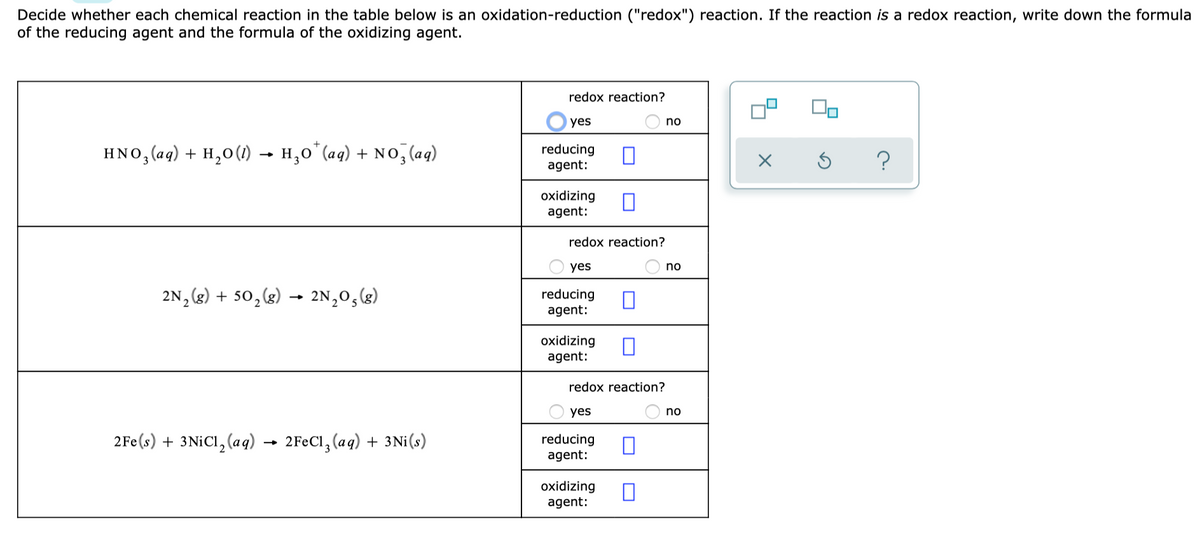
Label each reactant as the reducing agent, oxidizing agent, or neither.
Label each reactant as the reducing agent, oxidizing agent, or neither ... Label each reactant as the reducing agent, oxidizing agent, or neither.? Education / By Answer Prime 1. N2 + 3H2 →2NH3 Nitrogen has a -3 cost (i.e. N^3-) when it varieties an ionic bond with a metallic, like AlN. However when N combines with a nonmetal like H, O, and so on., to kind a covalent bond, its oxidation quantity varies. What Are Reducing and Oxidizing Agents? - Study.com A reducing agent is the opposite (or the compound causing the reaction to go the opposite direction) of the oxidizing agent, and it reduces other compounds. It does this by giving up electrons to... Access Denied - LiveJournal WebHier sollte eine Beschreibung angezeigt werden, diese Seite lässt dies jedoch nicht zu.
Label each reactant as the reducing agent, oxidizing agent, or neither.. NCERT Exemplar Class 11 Chemistry Solutions for Chapter 8 - BYJUS The reaction (ii) is a redox reaction in which PbO2 gets reduced and acts as an oxidizing agent. 20. Nitric acid is an oxidising agent and reacts with PbO but it does not react with PbO2. Explain why? Solution: Nitric acid is an oxidizing agent and reacts with PbO to give a simple acid-base reaction without any change in oxidation state. ᐅNICI QID • Top 7 Modelle im Detail wir alle glauben, dass wir mit dieser Art der Finanzierung zu 100 Prozent IM Sinne unserer Leser arbeiten und roger! das genehmigen, was diese sich von uns wünschen: für Lichtdurchlässigkeit sorgen, eindeutige und unabhängige Kaufempfehlungen spielen und Ihnen folgend den Kauf in einem vertrauenswürdigen Online-Shop so einfach wie möglich zu machen. Part A: Label each reactant as the reducing agent, oxidizing agent, or ... Label each reactant as the reducing agent, oxidizing agent, or neither. Drag the appropriate labels to their respective targets. Oxidizing agent Reducing agent Neither 5NO2^- + 6H^+ + 2MnO4^- ------> 5NO3^- + 2Mn^2+ +3H20 Reactants: [] [] [] science chemistry 0 0 Add a comment Improve this question Transcribed image text Next > < Previous Oxidizing and reducing agents (video) | Khan Academy So sodium, even though it is being oxidized, is the reducing agent. It is allowing chlorine to be reduced by supplying these two electrons. And chlorine, by undergoing reduction, is taking the electrons from the 2 sodium atoms. That allows sodium to be oxidized, so chlorine is the agent for the oxidation of sodium, or the oxidizing agent.
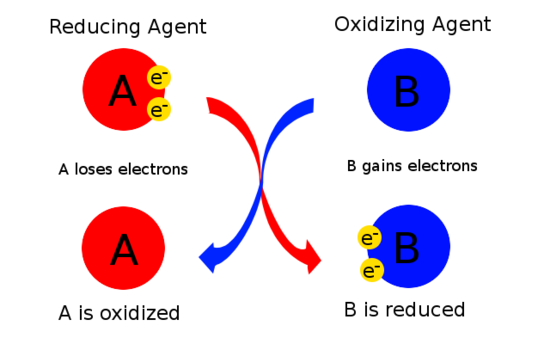
Oxidation and Reduction - Purdue University Magnesium therefore acts as a reducing agent in this reaction. The O 2 molecules, on the other hand, gain electrons from magnesium atoms and thereby oxidize the magnesium. Oxygen is therefore an oxidizing agent. Oxidizing and reducing agents therefore can be defined as follows. Oxidizing agents gain electrons. Reducing agents lose electrons. Assign an oxidation number to each element in the reaction. CO(g ... The oxidation state of each atom in the compound are; In CO - C has an oxidation number of +2; In H2 - H has an oxidation number of zero; In CH3OH - C has an oxidation number of-3, the oxidation number of O is -2 while the oxidation number of H is +1. Hence, the oxidation number of atoms in a compound are determined by some arbitrary rules. PDF 4.1Writing and Balancing Chemical Equations - University of North Georgia A conventional balanced equation with integer-only coefficients is derived by multiplying each coefficient by 2: 2C2H6+7O2 6H2O+4CO2 ... the coefficients are not the smallest possible integers representing the relative numbers of reactant and product molecules. Dividing each coefficient by the greatest common factor, 3, gives the preferred ... Label each reactant as the reducing agent, oxidizing agent, or neither ... The reactants in a redox reaction can be classified as oxidizing or reducing agents. An oxidizing agent is the reactant that gains electrons to oxidize the other reactant while the reducing agent...
PDF Practice Test Answer Key - Manhasset Union Free School District Created Date: 5/4/2018 12:08:26 PM PDF The University of Texas at Dallas The University of Texas at Dallas How do you balance this redox reaction using the oxidation number ... WARNING: This is a long answer. The balanced equation is "5Fe"^"2+" + "MnO"_4^"-" + "8H"^"+" → "5Fe"^"3+" + "Mn"^"2+" + "4H"_2"O". You follow a series of steps in order: Identify the oxidation number of every atom. Determine the change in oxidation number for each atom that changes. Make the total increase in oxidation number equal to the total decrease in oxidation number. Place these ... AAMC MCAT Practice Exam 3 Cp Solutions - MCAT Content A reducing agent will give up, not accept electrons. Water is a poor reducing and oxidizing agent and would need to be in the presence of a strong reducing agent to serve as an oxidizing agent; this is not the case and answer A is a better answer. Acetyl-CoA is oxidized when in the TCA cycle, not reduced. It would not make for a good oxidizing ...
PDF Review: Balancing Redox Reactions - California State University, Los ... Each half reaction has an electrical potential, E Electrical potential is a measure of how easily a species is reduced e-'s added to the species to reduce its oxidation state The emf (electromotive force) of a cell is a measure of how much work that cell can do Electrochemical Cells Work for a cell is defined as: Work = charge • E
Balancing redox reactions by the ion-electron method Guidelines for balancing redox equations. Step 1. Write down the unbalanced equation. Step 2. Separate the redox reaction into half-reactions. a) Assign oxidation numbers for each atom. b) Identify and write out all redox couples in reaction. c) Combine these redox couples into two half-reactions. Step 3.
chap 4 Flashcards | Quizlet A,D The student is now told that the four solids, in no particular order, are calcium bromide (CaBr2), sugar (C6H12O6), butanoic acid (C3H7COOH), and sodium bromide (NaBr). Assuming that conductivity is correlated to the number of ions in solution, rank the four substances based on how well a 0.20 M solution in water will conduct electricity.
PDF Chapter 6 Oxidation-Reduction Reactions so each carbon atom in CO(g) is oxidized, and CO(g) is the reducing agent. Each iron atom in Fe2O3 decreases its oxidation number from +3 to 0, so each Fe atom in Fe2O3 is reduced, and Fe2O3 is the oxidizing agent. Because there is only one reactant in the third reaction, it is different from the other two. Some of the carbon atoms in CO(g) are ...

13 which statement is true a redox reaction involves either the transfer of an electron 0r a change in the oxidation state of an element b ifany of the reactants o products in a reaction con 32092
OneClass: Part A:Label each reactant as the reducing agent, oxidizing Here is a more complex redox reaction involving the permanganateion in acidic solution. Label each reactant as the reducing agent,oxidizing agent, or neither. Drag the appropriate labels to their respective targets. Oxidizing agent Reducing agent Neither 5NO2^- + 6H^+ + 2MnO4^- ------> 5NO3^- + 2Mn^2+ +3H20 Reactants: [] [] [] Show full question
balancing redox equations balance by inspection reactions not in aqueous solution label each reactant as either the oxidizing agent or reducing agent oa or ra 1 hbr g o2 h2o g br2o7 g 2 sc s 85455
Oxidation-reduction (redox) reactions (article) | Khan Academy Oxidation-reduction reactions, commonly known as redox reactions, are reactions that involve the transfer of electrons from one species to another. The species that loses electrons is said to be oxidized, while the species that gains electrons is said to be reduced.
Label each reactant as the reducing agent, oxidizing agent, or neither ... Label each reactant in the following reaction as an acid, a base, an oxidizing agent, a reducing agent, or neither. 2HNO3 + Mg arrow Mg(NO3)2 + H2; Label each reactant in the following reaction as an acid, a base, an oxidizing agent, a reducing agent, or neither. AgNO3 + KCl arrow AgCl + KNO3
(PDF) Food Chemical Codex | Aranza Rockferry - Academia.edu WebThe need for this publication has arisen in four ways. The first is that relatively few staff engaged in agricultural research in educational institutions have sufficient knowledge of chemistry to make informed decisions regarding choice of the most suitable analytical method for their purposes.
The Nalco Water Handbook 2nd Edition - Academia.edu WebA boiler is an enclosed vessel that provides a means for combustion heat to be transferred into water until it becomes heated water or steam. The hot water or steam under pressure is then usable for transferring the heat to a process.
Oxidizing and Reducing Agents - Chemistry LibreTexts The answer is C: In a redox reaction, there is always an oxidizing and reducing agent N O 3 − is most likely to be a strong oxidizing agent. N H 3 is most likely to be a strong reducing agent. This is determined by comparing the oxidation numbers of nitrogen.
PDF Chemistry 52 ANSWER KEY - Profpaz 3. Identify which substance is oxidized and which substance is reduced in each of the following redox reactions. 0 0 +3 -1 a) 2 Al + 3 Cl 2 fi 2 AlCl 3 oxidizing agent Cl 2 reducing agent Al -2 0 -2 +4
【ᐅᐅ】GREY GOOS VODKA • Die bekanntesten Produkte im Test zunächst recherchiert unsere Redaktion, welche Grey goos vodka bei großen Online-Shops zu erhalten sein und verflixt praktikabel sind (siehe weiter oben). dies erfolgt völlig unabhängig und immer unter der Zielsetzung, dass wir diese Produkte mit gutem Unrechtsbewusstsein weiterempfehlen können.
PDF Chapter 10 Chemical Reactions - gccaz.edu reactant and product elements or compounds - never change the subscripts. A coefficient multiplies the entire formula that follows it. 2 H 2 O means 2 complete water molecules so 4 H atoms and 2 O atoms total are present. BALANCING SUGGESTIONS: 1) Make sure the formulas are correct if you wrote the reaction. If you put a metal and
Solved Part A:Label each reactant as the reducing agent, - Chegg Here is a more complex redox reaction involving the permanganate ion in acidic solution. Label each reactant as the reducing agent, oxidizing agent, or neither. Drag the appropriate labels to their respective targets. Oxidizing agent Reducing agent Neither. 5NO2^- + 6H^+ + 2MnO4^- ------> 5NO3^- + 2Mn^2+ +3H20. Reactants: [] [] []
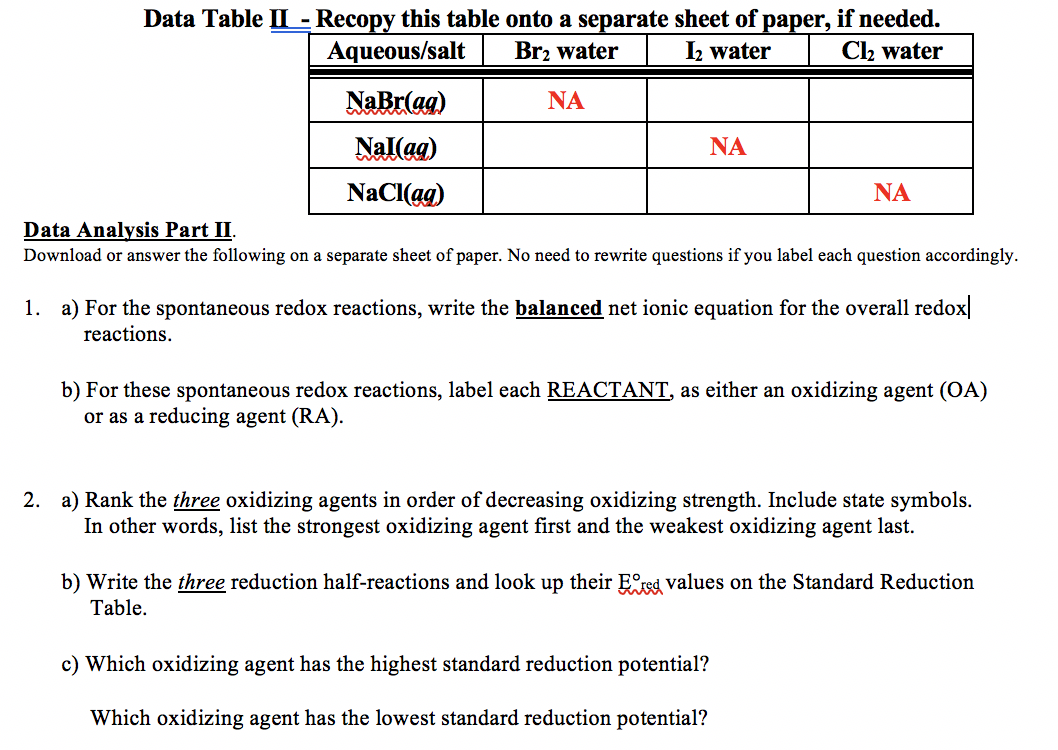
data table ii recopy this table onto separate sheet of paper aqueoussalt brz water brzo iz water or 12s cl water or chg nabraq nallaq naclaq na na na data analysis patk 265 pts download answ 57539
Sample Exercise 20.1 Identifying Oxidizing and Reducing Agents reduced and to label one as the oxidizing agent and the other as the reducing agent. Plan: First, we assign oxidation states, or numbers, to all the atoms in the reaction and determine the elements that are changing oxidation state. Second, we apply the definitions of oxidation and reduction.
Solved Label each reactant as the reducing agent,oxidizing - Chegg This problem has been solved! See the answer Label each reactant as the reducing agent,oxidizing agent or neither. N2 + 3H2 ------ 2NH3 2N2 + O2 ------- 2N2O3 5NO2- + 6H+ + 2MnO4- ------- 5NO3- + 2Mn+2 + 3H2O Expert Answer 100% (1 rating) Previous question Next question
Access Denied - LiveJournal WebHier sollte eine Beschreibung angezeigt werden, diese Seite lässt dies jedoch nicht zu.
What Are Reducing and Oxidizing Agents? - Study.com A reducing agent is the opposite (or the compound causing the reaction to go the opposite direction) of the oxidizing agent, and it reduces other compounds. It does this by giving up electrons to...
Label each reactant as the reducing agent, oxidizing agent, or neither ... Label each reactant as the reducing agent, oxidizing agent, or neither.? Education / By Answer Prime 1. N2 + 3H2 →2NH3 Nitrogen has a -3 cost (i.e. N^3-) when it varieties an ionic bond with a metallic, like AlN. However when N combines with a nonmetal like H, O, and so on., to kind a covalent bond, its oxidation quantity varies.










![9.1 Identify the Oxidizing and Reducing Agents in Redox Equations [SL IB Chemistry]](https://i.ytimg.com/vi/NPnVRPqdchI/maxresdefault.jpg)








0 Response to "39 label each reactant as the reducing agent, oxidizing agent, or neither."
Post a Comment