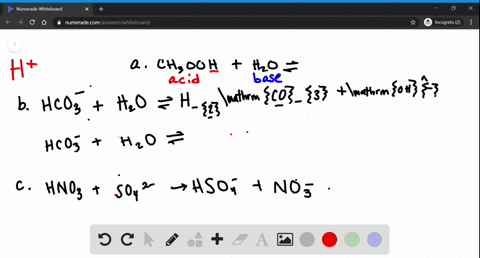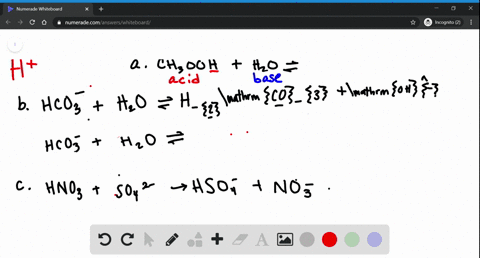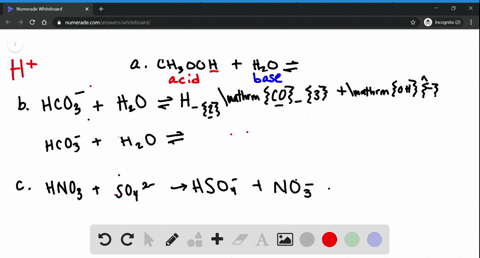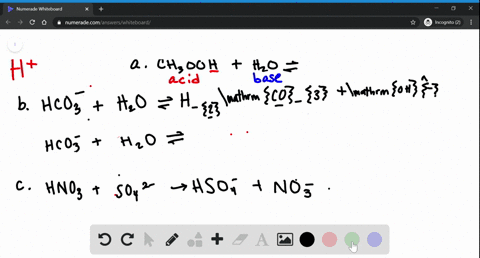
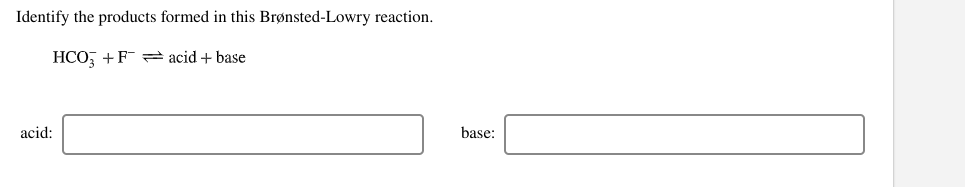
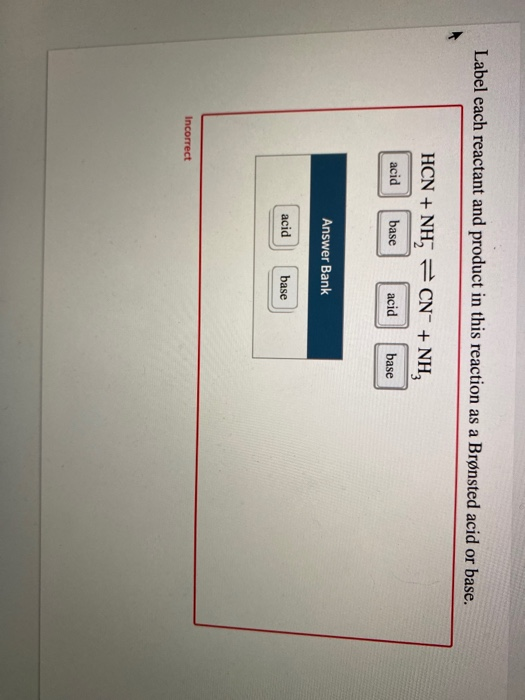
39 label each reactant and product as a bronsted acid or base
Answered: 2. For each of the following acid-base… | bartleby Oct 08, 2022 · For each of the following acid-base reactions, predict the products. Determine whether the reaction is favored as written. Label the acid, base, conjugate acid and conjugate base. a. CH₂CH=CH₂ NaNH, b. C. d. CH₂ONa NaOH NaNH, + + … solomons, graham - fundamentals of organic chemistry.pdf solomons, graham - fundamentals of organic chemistry.pdf - Academia.edu ... chemistry
Assignment Essays - Best Custom Writing Services Get 24⁄7 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply.

Label each reactant and product as a bronsted acid or base
Chemistry Notes Form 4 - Chemistry Form Four Pdf - Online … It is a Bronsted-Lowry conjugate base. Every base /acid from Bronsted-Lowry definition thus must havea conjugate product/reactant. II. From the equation: HCl(aq) + NH 3 aq) === NH 4 +(aq) + Cl-(aq) (a)(i) For the forward reaction from left to right, NH 3 gains a proton to form NH 4 + and thus NH 3 is a proton acceptor . It is a Bronsted-Lowry base General Chemistry 1 PDF | PDF | Gases | Molecules - Scribd Bronsted acids and bases to the pH of 2. discuss the acid-base property of water STEM_GC11AB-IVf-g-154 2. The acid-base properties of solutions and the water use of buffer 3. define pH STEM_GC11AB-IVf-g-155 3. pH- a measure of acidity solutions 4. 13.1 Acids and bases | Types of reactions | Siyavula Conjugate acid-base pairs. Using the common acids and bases in Table 13.1, pick an acid and a base from the list. Write a chemical equation for the reaction of these two compounds. Now identify the conjugate acid-base pairs in your chosen reaction.
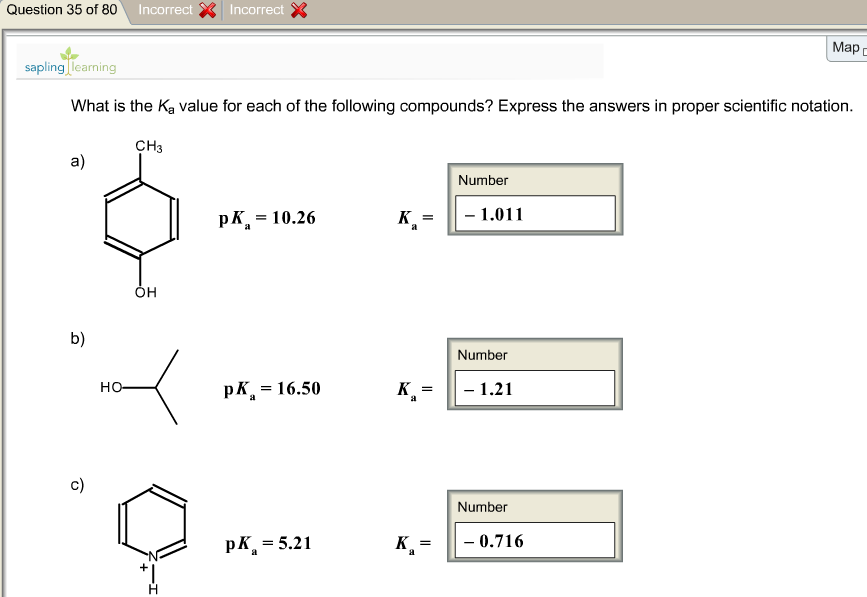
Label each reactant and product as a bronsted acid or base. Success Essays - Assisting students with assignments online Get 24⁄7 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply. Lewis Concept of Acids and Bases - Chemistry LibreTexts Aug 15, 2020 · Acids and bases are an important part of chemistry. One of the most applicable theories is the Lewis acid/base motif that extends the definition of an acid and base beyond H + and OH-ions as described by Br ø nsted-Lowry acids and bases. The Brø nsted acid-base theory has been used throughout the history of acid and base chemistry. However ... (PDF) Engineering Chemistry by Jain & Jain - Academia.edu As we know that matter exists in different physical states under different conditions of temperature and pressure. For example solid state, liquid gases plasma and BEC etc. Inorganic Chemistry By GARY L. MIESSLER - Academia.edu From very early times, alchemists gave names to substances, although these names gave little if any indication of the actual composition and or structure, which is the aim of a true nomenclature.
13.1 Acids and bases | Types of reactions | Siyavula Conjugate acid-base pairs. Using the common acids and bases in Table 13.1, pick an acid and a base from the list. Write a chemical equation for the reaction of these two compounds. Now identify the conjugate acid-base pairs in your chosen reaction. General Chemistry 1 PDF | PDF | Gases | Molecules - Scribd Bronsted acids and bases to the pH of 2. discuss the acid-base property of water STEM_GC11AB-IVf-g-154 2. The acid-base properties of solutions and the water use of buffer 3. define pH STEM_GC11AB-IVf-g-155 3. pH- a measure of acidity solutions 4. Chemistry Notes Form 4 - Chemistry Form Four Pdf - Online … It is a Bronsted-Lowry conjugate base. Every base /acid from Bronsted-Lowry definition thus must havea conjugate product/reactant. II. From the equation: HCl(aq) + NH 3 aq) === NH 4 +(aq) + Cl-(aq) (a)(i) For the forward reaction from left to right, NH 3 gains a proton to form NH 4 + and thus NH 3 is a proton acceptor . It is a Bronsted-Lowry base

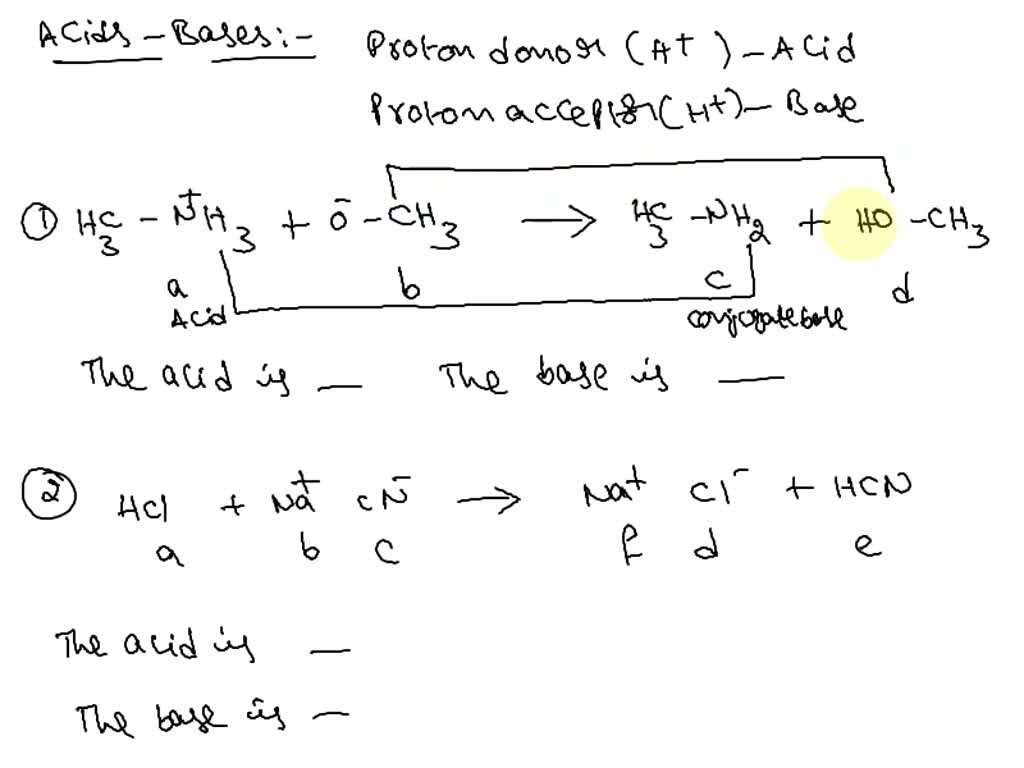
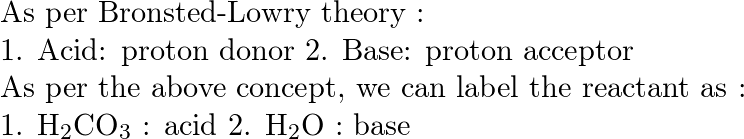
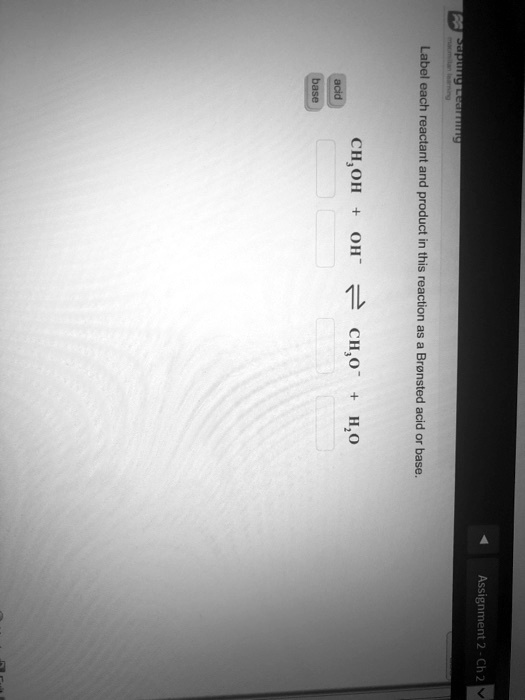

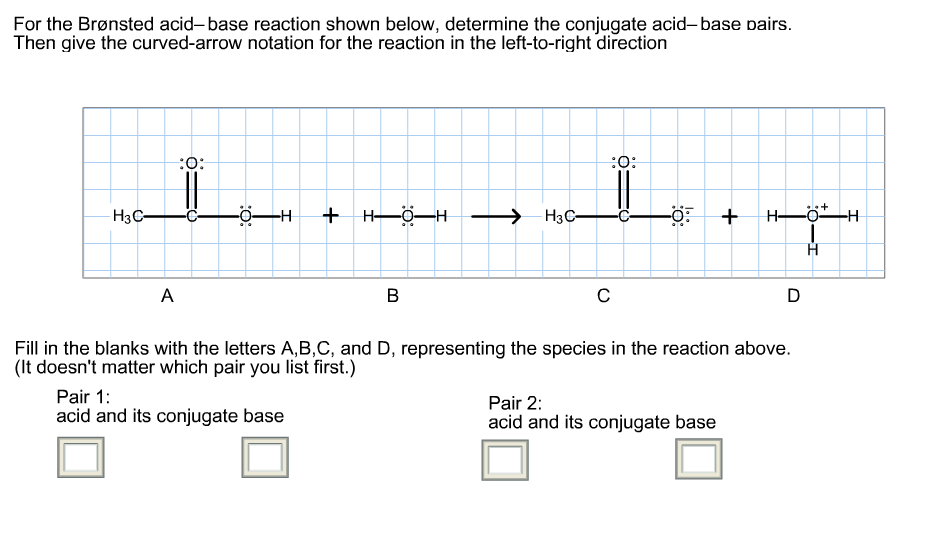
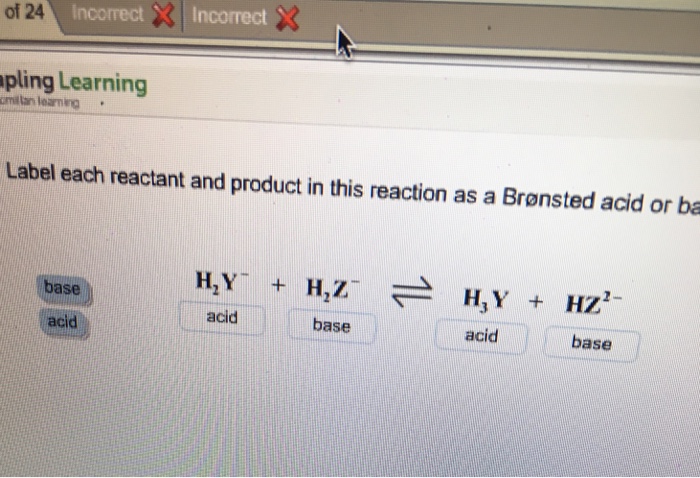
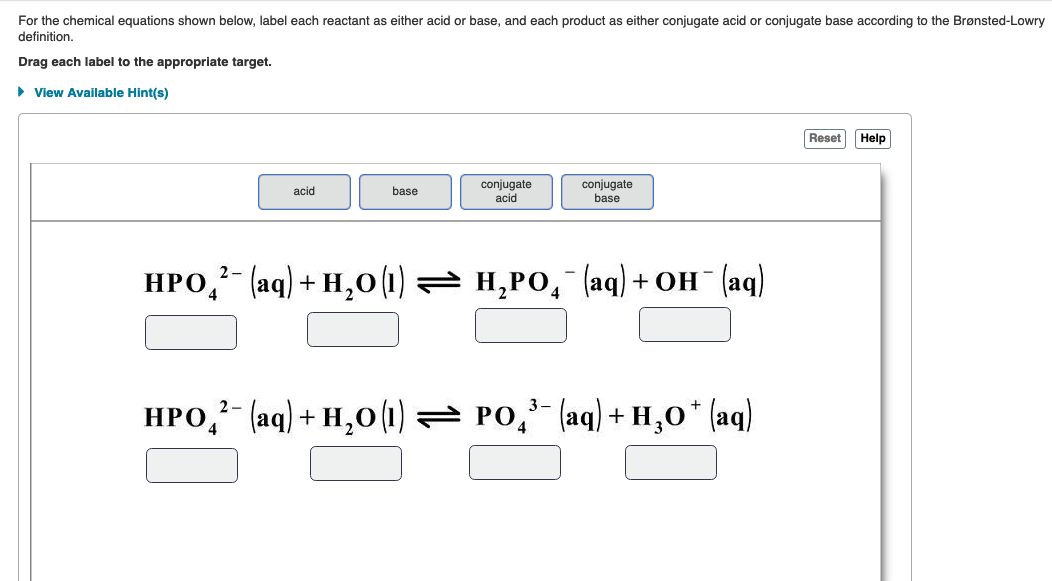
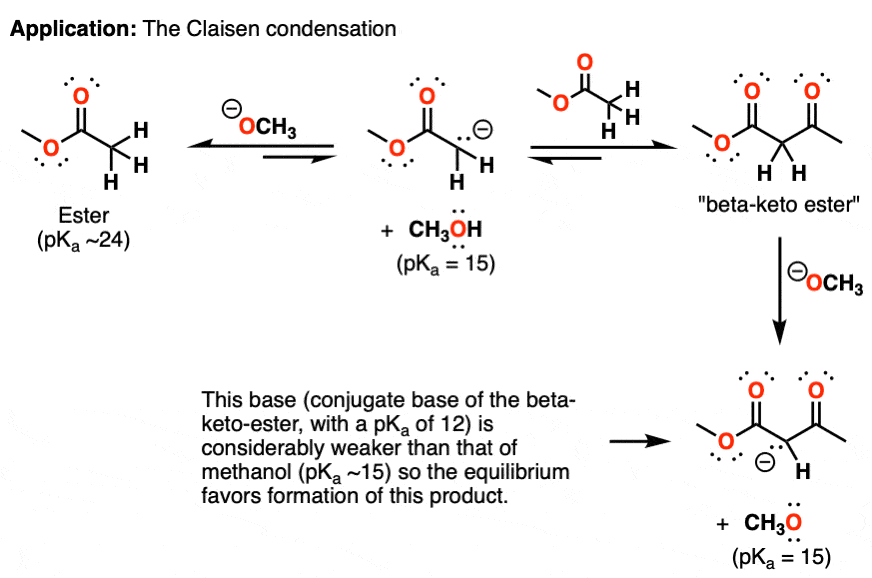




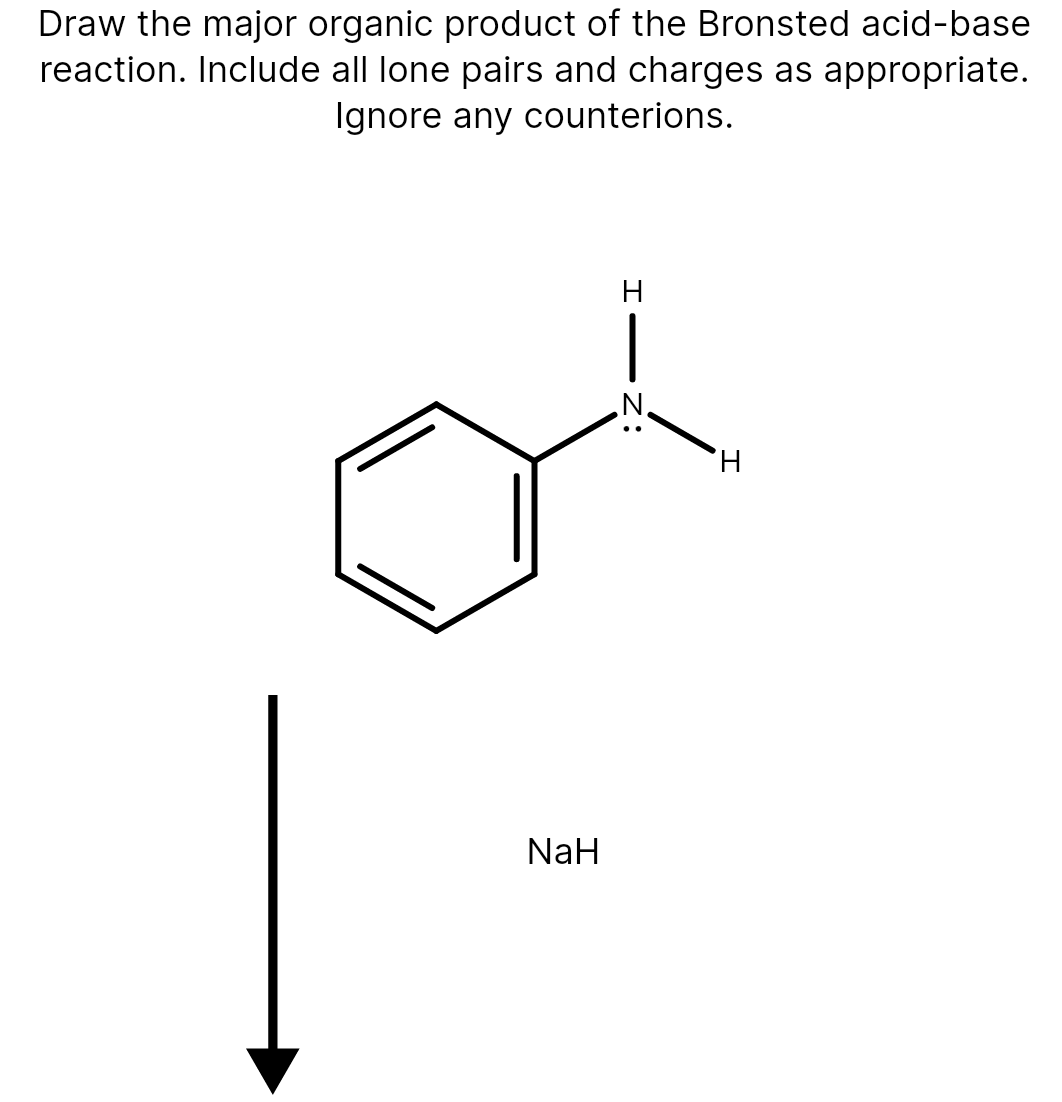
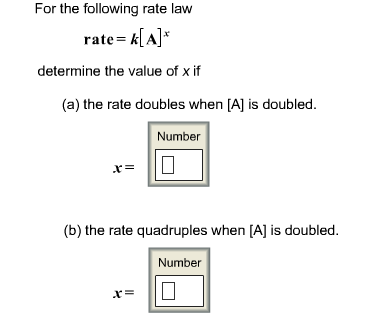
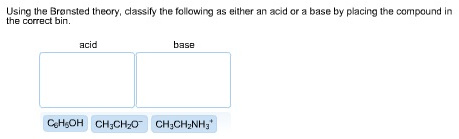



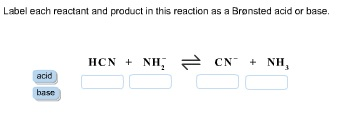



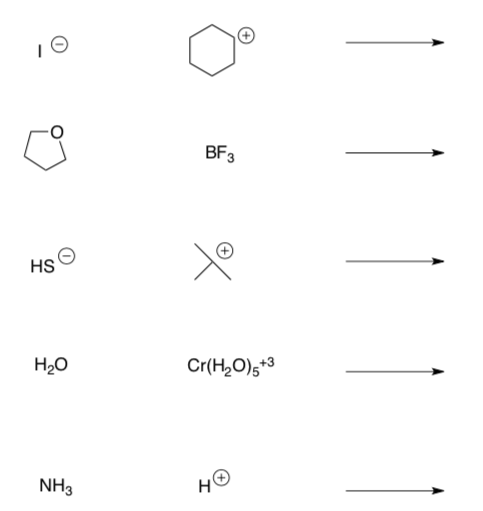
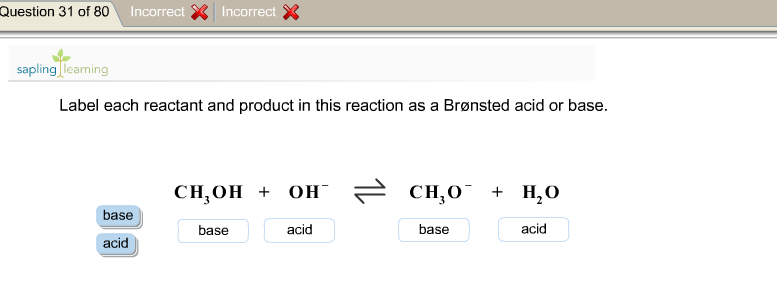


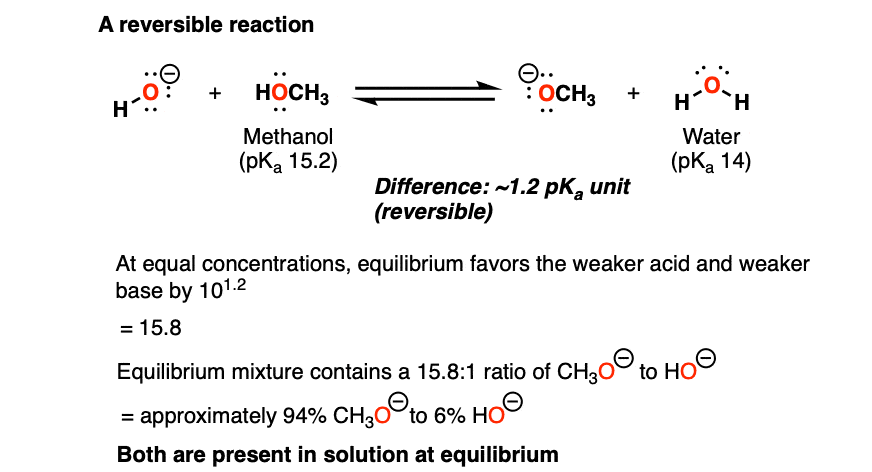
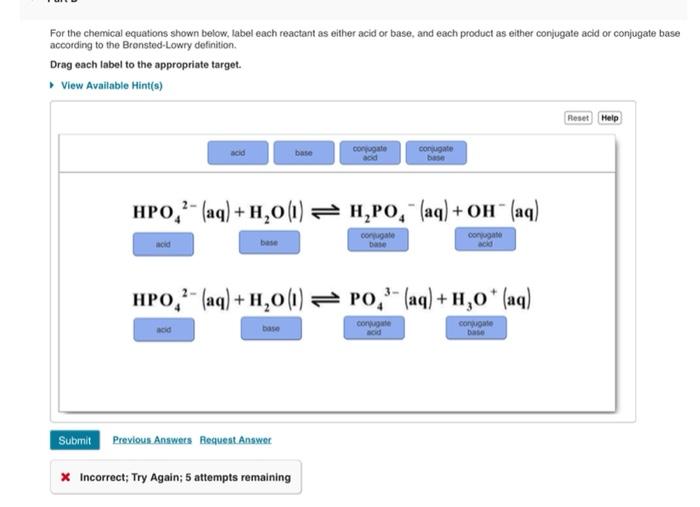
0 Response to "39 label each reactant and product as a bronsted acid or base"
Post a Comment