41 medical device label symbols
EUR-Lex - 32017R0746 - EN - EUR-Lex - Europa ‘ in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software or system, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and ... EU MDR – Medical Device Labeling Changes & Challenges ... Sep 08, 2021 · Global rollout of EU MDR and other UDI-type of regulations are driving all medical device companies to revisit their labeling processes to ensure they are all compliant across the extended supply chain. The European Medical Device Regulations (MDR) 2017/745 and In Vitro Diagnostic Regulations (IVD) 2017/746 were published on May 5, 2017 in the Official […]
Medical Device Marking and Labeling - mddionline.com May 01, 2004 · In a global marketplace, harmonization of graphic symbols eases the language barriers and lessens the potential for device misuse. A review of IEC 60601-1's global regulatory importance, "A Primer for IEC 60601-1," was presented in an earlier article on MD+DI, and the differences between national standards were discussed in "National Deviations to IEC 60601-1."
Medical device label symbols
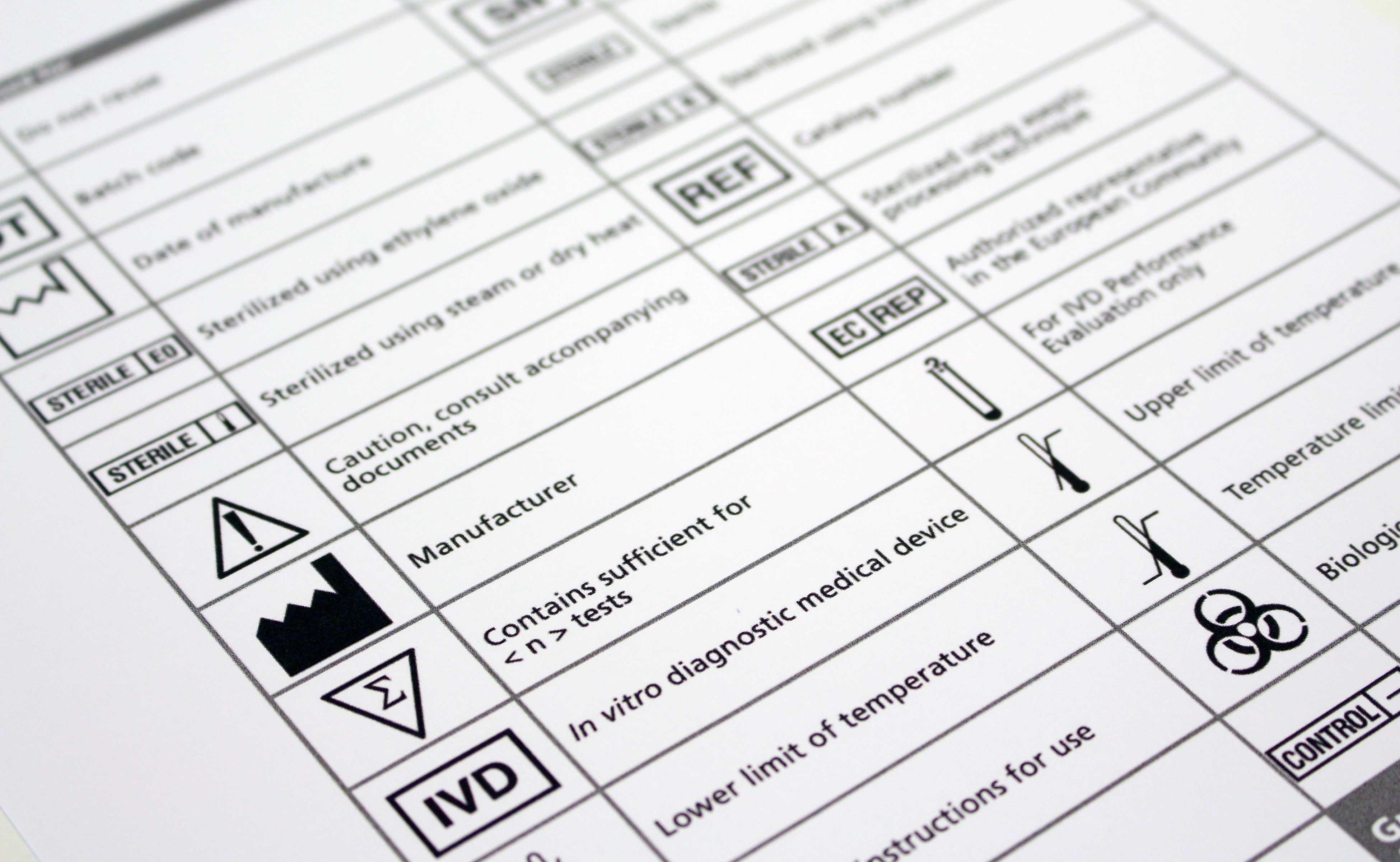
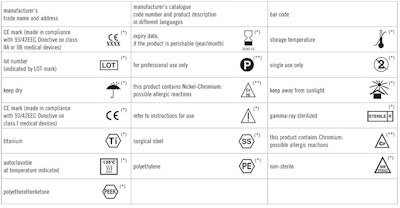
Medical Device Label Symbols Glossary - Cardinal Health Symbol Glossary for Medical Device Labels. eCFR :: 21 CFR Part 801 -- Labeling Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the device, the date must be presented in the following format: The year, using four digits; followed by the month, using two digits; followed by the day, using two digits; each ... Guidance Document: Guidance for the Labelling of Medical … 12/06/2004 · Each device including a system, medical device group, medical device family, or medical device group family must have a name. The device licence is issued for (a) the device name on the label which may describe one device, (b) an administrative grouping of devices sold for convenience under a single name or (c) a grouping of devices that carry the same generic …
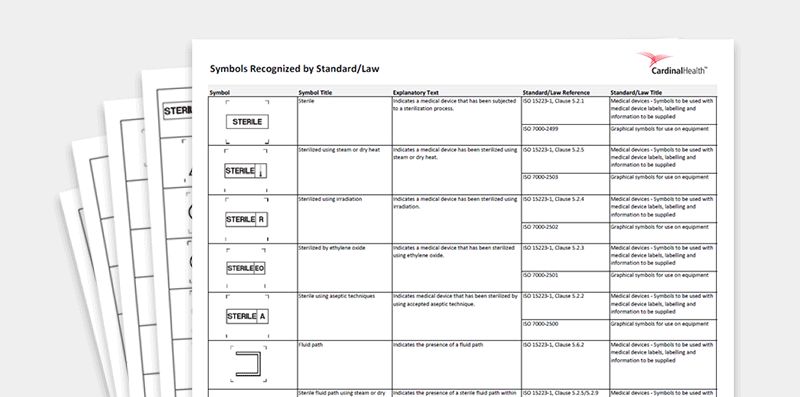
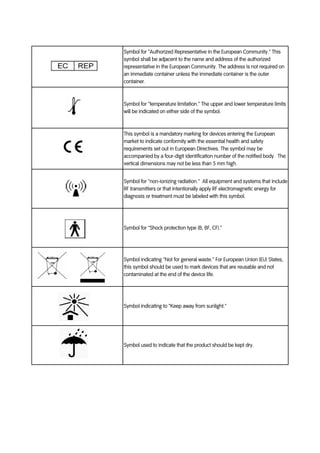
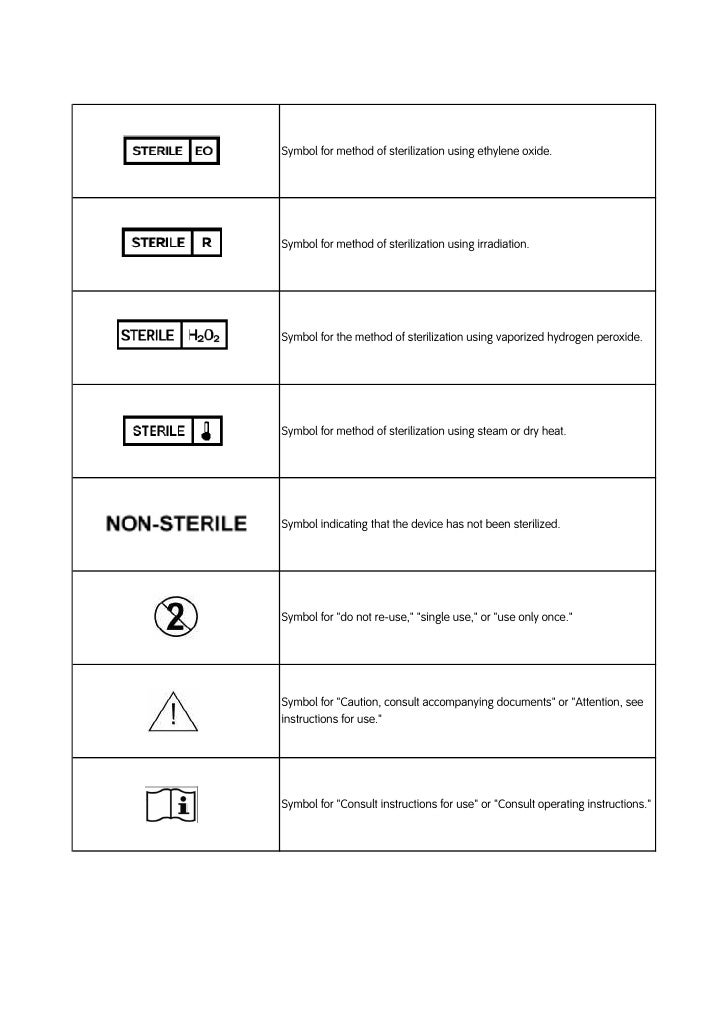
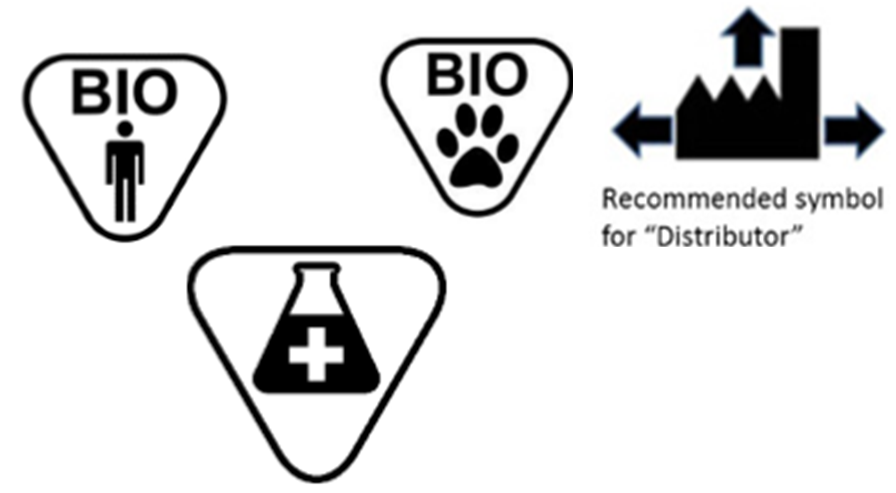
Medical device label symbols. General Specifications Change Notifications (GSCN ... Update the General Specification to enable Direct Part Marking to be applied to all medical devices and enable use of smaller GS1 DataMatrix symbols than currently permitted for medical and surgical instruments. GSCN for 14-005 Updates for GS1 Logistics Label Acronyms and glossary terms | Therapeutic Goods ... Active medical device A medical device that is intended by the manufacturer: to depend for its operation on a source of electrical energy or other source of energy (other than a source of energy generated directly by a human being or gravity); and to act by converting this energy; but does not include a medical device that is intended by the manufacturer to transmit energy, a substance, or any ... How to properly label a medical device according to the MDR ... a mandatory information that a given product is a medical device, or obligatory use of harmonized symbols; placing the UDI code carrier on the device label. Placing the UDI code on the labels is not obligatory as of the date of entry into force of the MDR Regulation for most classes of medical devices. The document provides for a transition ... Glossary of standard symbols | Cook Medical Symbols to be used with medical device labels, labelling, and ... Symbol. Standard. Ref #. Title. Description. ISO 15223-1. Medical devices –.
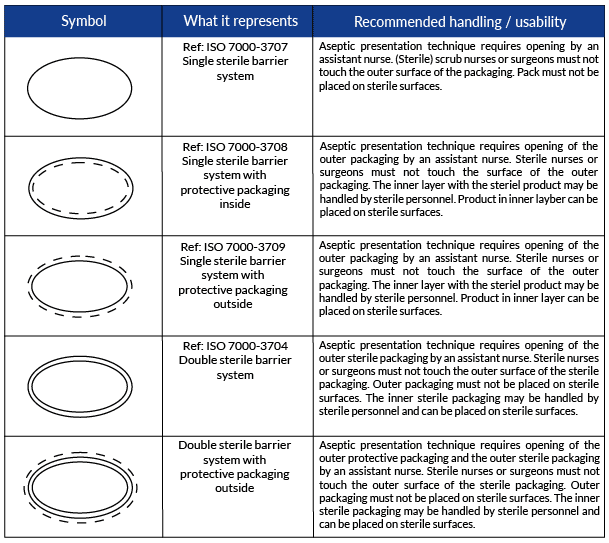
Symbols to be used on labelling (ISO 15223) & Information to ... - BSI This symbol shall be accompanied by the name and address of the manufacturer (i.e. the person placing the medical device on the market), adjacent to the symbol. Symbols Commonly Used in Medical Device and Packaging Labeling Symbols Commonly Used in Medical Device and Packaging Labeling. PAGE 1. No. Symbol. Name. Description. Standard. Clause. 1. Rx only. Caution: Federal law. Medical Device Symbols | I3CGLOBAL EN ISO 15223-1:2012: Medical devices Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. Medical Device Symbols used in Active & Non Active Devices EUR-Lex - 01993L0042-20071011 - EN - EUR-Lex - Europa Oct 11, 2007 · (c) ‘ in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, equipment or system, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations,
ISO 15223-1:2016 - Medical devices — Symbols to be used with ... ISO 15223-1:2016 identifies requirements for symbols used in medical device labelling that convey information on the safe and effective use of medical ... eCopy Program for Medical Device Submissions | FDA 23/06/2022 · An electronic copy (eCopy) is an electronic version of your medical device submission stored on a compact disc (CD), digital video disc (DVD), or a flash drive. Including an eCopy with your ... Applications for medical devices under the Interim Order for … COVID-19 medical device: A medical device that is manufactured, sold or represented for use in relation to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Control number: A unique series of letters, numbers or symbols, or any combination of these, that is assigned to a medical device by the manufacturer and from which a history of the manufacture, packaging, … Medical device labeling checklist - pzl.raskhodchikov.info EN ISO 15223 -1:2012: Medical devices Symbols to be used with medical device labels, labeling, and. 13.1 Each device must be accompanied by the information needed to use it safely and properly, taking account of the training and knowledge of the potential users 23.1(a) The medium, format, content, legibility, and location of the label and instructions for use shall be …
EUR-Lex - 01993L0042-20071011 - EN - EUR-Lex - Europa 11/10/2007 · Where a device incorporates, as an integral part, a human blood derivative, the notified body shall, having verified the usefulness of the substance as part of the medical device and taking into account the intended purpose of the device, seek a scientific opinion from the EMEA, acting particularly through its committee, on the quality and safety of the substance …
IFU for Medical Devices, a Definitive Guide (EU & US) - INSTRKTIV 25/05/2022 · How to format dates provided on a medical device label. Let’s have a closer look at all of these requirements. Provide the name and address of the manufacturer on the label . The label of a medical device in package form must state the name and place of business of the manufacturer, packer, or distributor. When a corporation places the medical device on the …
Symbols Glossary - ICU Medical ISO 15223-1 Reference #5.1.2. FDA Recognition # 5-117. Medical devices - Symbols to be used with medical device labels, labelling and information to be.
Symbols | Mölnlycke The unique device identification (UDI) is a unique numeric or alphanumeric code related to a medical device. It allows for a clear and unambiguous ...
Use of Symbols to Indicate Compliance with the MDR The Medical Devices Regulation 2017/745/EU ('MDR') has new requirements that ask for various kinds of information to be indicated on the label of medical ...
How to properly label a medical device according to the MDR … a mandatory information that a given product is a medical device, or obligatory use of harmonized symbols; placing the UDI code carrier on the device label. Placing the UDI code on the labels is not obligatory as of the date of entry into force of the MDR Regulation for most classes of medical devices. The document provides for a transition ...
EUR-Lex - 32017R0746 - EN - EUR-Lex - Europa Upon a duly substantiated request of a Member State, the Commission shall, after consulting the Medical Device Coordination Group established under Article 103 of Regulation (EU) 2017/745 (MDCG), by means of implementing acts, determine whether or not a specific product, or category or group of products, falls within the definitions of ‘ in vitro diagnostic medical device’ or …
Symbol Glossary Definitions - BD Mar 3, 2022 ... 5.1.2, Medical devices — Symbols to be used with information to be supplied by ... A.4, Symbol for use in the labeling of medical devices ...
Symbol Glossary Definitions - Medtronic Diabetes Manufacturer Indicates the medical device manufacturer. ISO 15223-1, Clause 5.1.1 Medical devices — Symbols to be used with medical device labels, labelling ...
Guidance Document: Guidance for the Labelling of Medical … 12/06/2004 · Each device including a system, medical device group, medical device family, or medical device group family must have a name. The device licence is issued for (a) the device name on the label which may describe one device, (b) an administrative grouping of devices sold for convenience under a single name or (c) a grouping of devices that carry the same generic …
eCFR :: 21 CFR Part 801 -- Labeling Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the device, the date must be presented in the following format: The year, using four digits; followed by the month, using two digits; followed by the day, using two digits; each ...
Medical Device Label Symbols Glossary - Cardinal Health Symbol Glossary for Medical Device Labels.














0 Response to "41 medical device label symbols"
Post a Comment