41 a solution contains the label 0.2 m kno3. what is the correct interpretation of this concentration?
CHEM Final Review Flashcards | Quizlet A student needs to prepare 250.0 mL of a 2.50 M HCl solution using the stock solution 12.0 M HCl. What volume of 12.0 M HCl is required for this dilution? 52.1 mL. A solution contains the label 0.2 M KNO3. What is the correct interpretation of this concentration? There are 0.2 moles of KNO3 in 1 L of solution. › 18218712 › The_Nalco_WaterThe Nalco Water Handbook 2nd Edition - Academia.edu The solubility of a gas in a liquid is expressed by Henry's Law: Ctotal = kP where: Ctotal = total concentration of the gas in solution P = partial pressure of the gas above solution k = a proportionality constant known as Henry's Law Constant For example, 8 ppm of oxygen can be dissolved in water when the partial pressure of oxygen is 0.2 ...
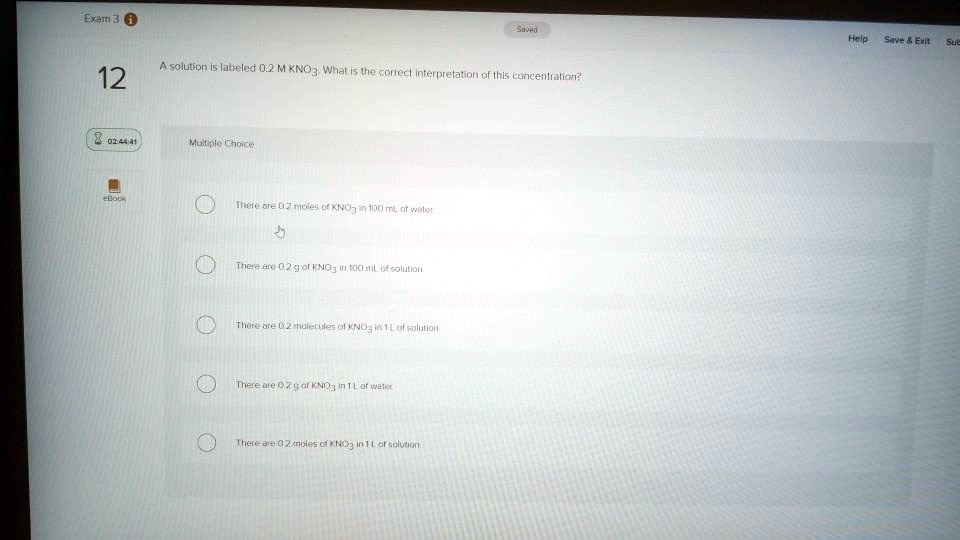
A solution contains the label 0.2 M KNO3. What is the correct ... A solution contains the label 0.2 M KNO3. What is the correct interpretation of this concentration? O3 in 100 mL of water. O There are 0.2 g of KNO3 in 1 L of water. O There are 0.2 molecules of KNO3 in 1 L of solution. O There are 0.2 g of KNO3 in 100 mL of solution. O There are 0.2 moles of KNO3 in 1 L of solution.
A solution contains the label 0.2 m kno3. what is the correct interpretation of this concentration?
Solved A solution contains the label 0.2 M KNO_3. What is - Chegg Expert Answer. Answer B t …. View the full answer. Transcribed image text: A solution contains the label 0.2 M KNO_3. What is the correct interpretation of this concentration? (PDF) Contrasting Demographics of Nontraditional Students in Two Off ... The Academic Program at Fort Lauderdale (APF), founded in 1984, and the Academic Program at Homestead (APH), founded in 2000, were established to enable place bound students to earn the Univ. of Fla. B.S. degree in horticulture. Although both Principles of Modern Chemistry - PDF Free Download - Donuts Electrochemistry 763 17.1 Electrochemical Cells 764 17.2 Cell Potentials and the Gibbs Free Energy 770 17.3 Molecular Interpretation of Electrochemical Processes 780 17.4 Concentration Effects and the Nernst Equation 781 17.5 Molecular Electrochemistry 787 17.6 Batteries and Fuel Cells 800 17.7 Corrosion and Corrosion Prevention 808 17.8 ...
A solution contains the label 0.2 m kno3. what is the correct interpretation of this concentration?. Argument-driven Inquiry In Chemistry : Lab Investigations For Grades 9 ... E-Book Overview Transform your chemistry labs with this guide to argument-driven inquiry. Designed to be much more authentic for instruction than traditional laboratory activities, the investigations in this book give high school students the opportunity to work the way scientists do. Chapter 6: Reactions and Solutions - Studylib A solution contains the label 0.2 M KNO3. What is the correct interpretation of this concentration? A) There are 0.2 moles of KNO3 in 100 mL of water. B) There are 0.2 g of KNO3 in 100 mL of solution. C) There are 0.2 molecules of KNO3 in 1 L of solution. D) There are 0.2 g of KNO3 in 1 L of water. E) There are 0.2 moles of KNO3 in 1 L of ... Chemistry Exam 2 Flashcards | Quizlet Study with Quizlet and memorize flashcards containing terms like Which statement concerning solutions is FALSE?, Which statement concerning solution concentration is FALSE?, A solution contains the label 0.2 M KNO3. What is the correct interpretation of this concentration? and more. dokumen.pub › general-chemistry-principles-andGeneral Chemistry: Principles and Modern Applications (10th ... It is a good idea to memorize the most common SI prefixes (such as G, M, k, d, c, m, m, n, and p) because you can t survive in a world of science without knowing the SI prefixes. official spelling of this unit is metre, but we will use the American spelling. mole is introduced in Section 2-7. cElectric current is described in Appendix B and in ...
CHEM Final Review Exam 4 Flashcards | Quizlet A solution contains the label 0.2 M KNO3. What is the correct interpretation of this concentration? There are 0.2 moles of KNO3 in 1 L of solution. The boiling point of pure benzene is 80.1oC. Suppose you had a solution of naphthalene in benzene. What will happen to the boiling point? Cambridge O Level Chemistry (Bryan Earl, Doug Wilford) iodide solution is added to a solution of iron(III) solution followed by the starch indicator, then a Key definitions blue-black colour will be seen as shown in Figure 3.11. This shows that the iron(III) ions must have Oxidation involves an increase in oxidation number. been reduced to iron(II) ions as the iodide ions are Reduction involves a ... › 43095131 › Fundamentals_ofFundamentals of Analytical Chemistry- 9th Edition - Academia.edu Polyfunctional acids and bases play important roles in many chemical and biological systems. The human body contains a complicated system of buffers within cells and within bodily fluids, such as human blood. Shown here is a scanning electron micrograph of red blood cells travel-ing through an artery. › 35126326 › Inorganic_ChemistryInorganic Chemistry (Atkins, Shriver).PDF - Academia.edu From very early times, alchemists gave names to substances, although these names gave little if any indication of the actual composition and or structure, which is the aim of a true nomenclature.
Maghemite Functionalization for Antitumor Drug Vehiculization Enter the email address you signed up with and we'll email you a reset link. › 32676640 › Cambridge(PDF) Cambridge International AS and A Level Chemistry ... Elements are organized into 18 vertical columns, or groups, and 7 horizontal rows, or periods. The two groups on the left and the six on the right are the main groups; the ten in the middle are the transition metal groups. › 48903430 › Inorganic_Chemistry_4Inorganic Chemistry 4th edition, Catherine Housecroft Purple acid phosphatases (PAPs) are a group of metallohydrolases that contain a dinuclear Fe(III)M(II) center (M(II) = Fe, Mn, Zn) in the active site and are able to catalyze the hydrolysis of a variety of phosphoric acid esters. Science/Math Questions & Answers | Transtutors A 20.00 mL sample of aqueous oxalic acid, H 2 C 2 O 4 was titrated with a 0.09113 M solution of potassium permanganate, KMnO 4 : 2푀푛푂 4 - ( (푎푞) + 5 퐻 2 퐶 2 푂 4...
Chap_6_Practice_Test - Chapter 6 Practice Test 69 Which... View Chap_6_Practice_Test from CHEM 1102 at University of Alabama. Chapter 6 Practice Test 69 Which statement concerning solution concentration is TRUE? If a bottle is labeled 0.10 M Ba(OH)2, the
› 43392556 › Cambridge_IGCSECambridge IGCSE Biology Third Edition Hodder Education Enter the email address you signed up with and we'll email you a reset link.
Special Project 1 - Improvised Manufacture of Nitric Acid PDF [ A u g u s t 2 3 , 2 0 0 2 , 0 4 : 4 4 P M : M e s s a g e e d i t e d b y : x o o 1 2 4 6 ] < / s m a l l > ... stanfield: Actually there should be Na2SO4 but I see what you mean. Given that the equation is correct, and given your numbers, you w ... I found this info on the effects of increasing H2SO4 concentration: In solution ...
(PDF) Using Fax-on-demand to Disseminate Agricultural Production ... Fax-on-demand is a new system of communications that combines computer, fax, and telephone technologies. Corporate use of fax-on-demand has shown it to be a rapid, user-friendly, economical way to disseminate technical support information. A project
Chemistry Question And Answers - Essay Help Here is the question: A 1.0 M HF solution is only 2.6 percent ionized. What is the K a value for HF?" If the answer was cut of… I selected the answer to the question because i cant select the question. Here is the question: "Calculate the percent ionization in a 1.0 M solution of nitrous acid, HNO2" Assignment on Redox Reactions.
Current Protocols Essential Laboratory Techniques Figure 2.1.9 Spectra of six dipicolinic acid (DPA) standard solutions. The absorbance at every wavelength is proportional to the concentration of the solution. Table 2.1.3 Concentration and Absorbance Values for Six Dipicolinic Acid Solutions Solution no. Concentration (M) Absorbance d1 1.010 × 10−4 0.477 d2 −5 0.324 −5 0.220 −5 0.150 ...
CHE 101-Ch 6 Homework Flashcards | Quizlet A solution contains the label 0.2 M KNO3. What is the correct interpretation of this concentration? There are 0.2 moles of KNO3 in 1 L of solution. Which diagram best represents the composition of an aqueous calcium chloride (CaCl2) solution? (water molecules are not shown)
Oxtobybook | Asifa Basharat - Academia.edu (Northern Arizona University) and Raymond Chang, this success guide is written for use with General Chemistry. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems. Solutions for all of the text's even-numbered problems are included.
Solved A solution contains the label 0.2 M KNO3. What is the - Chegg There are 0.2 g of KNO3 in 1 L of water. There are 0.2 moles of KNO3 in 1 L of solution. Question: A solution contains the label 0.2 M KNO3. What is the correct interpretation of this concentration? There are 0.2 moles of KNO3 in 100 mL of water. There are 0.2 g of KNO3 in 100 mL of solution. There are 0.2 molecules of KNO3 in 1L of solution ...
Encyclopedia Of Physical Science And Technology - Inorganic Chemistry ... The unique ability of inorganic ions to contain multiple unpaired spins means that MRI contrast agents are exclusively inorganic compounds. G. Chelation Therapy When the concentration of an inorganic ion is above the level where the body cannot return it to homeostasis through genetically controlled regulatory pathways, the ion is toxic.
Principles of Modern Chemistry - PDF Free Download - Donuts Electrochemistry 763 17.1 Electrochemical Cells 764 17.2 Cell Potentials and the Gibbs Free Energy 770 17.3 Molecular Interpretation of Electrochemical Processes 780 17.4 Concentration Effects and the Nernst Equation 781 17.5 Molecular Electrochemistry 787 17.6 Batteries and Fuel Cells 800 17.7 Corrosion and Corrosion Prevention 808 17.8 ...
(PDF) Contrasting Demographics of Nontraditional Students in Two Off ... The Academic Program at Fort Lauderdale (APF), founded in 1984, and the Academic Program at Homestead (APH), founded in 2000, were established to enable place bound students to earn the Univ. of Fla. B.S. degree in horticulture. Although both
Solved A solution contains the label 0.2 M KNO_3. What is - Chegg Expert Answer. Answer B t …. View the full answer. Transcribed image text: A solution contains the label 0.2 M KNO_3. What is the correct interpretation of this concentration?













0 Response to "41 a solution contains the label 0.2 m kno3. what is the correct interpretation of this concentration?"
Post a Comment