44 label each element in the chemical reaction below with the correct oxidation state.
SmartBook Ch. 4 Pt. IV Flashcards | Quizlet Match each species involved in a redox reaction with its correct description. 1) The species oxidized shows a(n) 2) The species reduced shows a(n) 3) The species that shows a decrease in oxidation number is the 4) The species that shows an increase in oxidation number is the a) reducing agent. b) oxidizing agent. c) increase in oxidation number. 20220316_130246.jpg - chemical reaction below Label each element in the ... View 20220316_130246.jpg from CHEM 121 at Unitek College, Fremont. chemical reaction below Label each element in the chemical reaction below with the correct oxidation. Study Resources. Main Menu; by School; by Literature Title; by Subject; by Study Guides; Textbook Solutions Expert Tutors Earn. Main Menu;
› resources › paper-101Paper Making Glossary: Your Guide to Paper Terminology A light duplication of a printed image on the other side of the same sheet, created by chemical reaction by the ink during the drying stages; also referred to as "Gas ghosting". Chemical Pulp Wood fiber cooked using chemicals producing a pulp used to manufacture numerous printing papers and paperboard products.
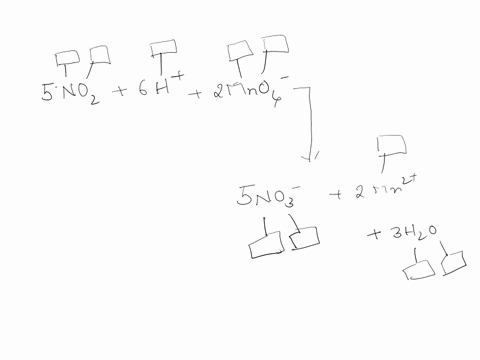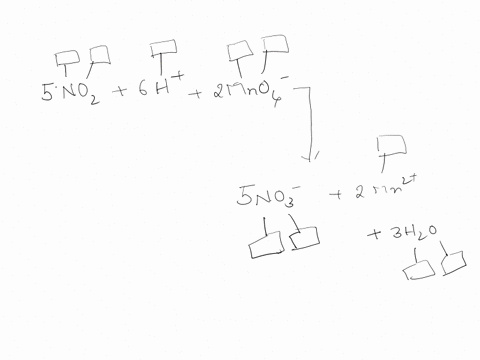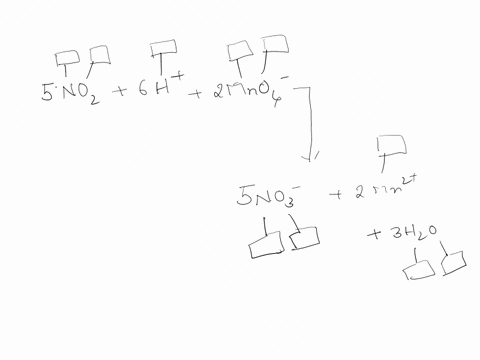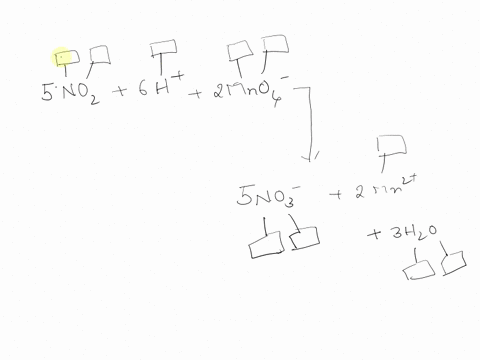
Label each element in the chemical reaction below with the correct oxidation state.
Redox Reactions - Examples, Types, Applications, Balancing - BYJUS The oxidation and reduction reactions always occur simultaneously in redox or Oxidation-Reduction reactions. The substance getting reduced in a chemical reaction is known as the oxidizing agent, while a substance that is getting oxidized is known as the reducing agent. JEE Main 2021 LIVE Chemistry Paper Solutions 24-Feb Shift-1 Memory-Based › publication › 241210010(PDF) Handbook of Monochromatic XPS Spectra - ResearchGate Jan 01, 1999 · Chemical Abstract Services number, common name, and the Latin language name of the element if available. The second page of each set is the wide scan survey spectrum with peak labels for each of ... Chem chapter 10 Practice Flashcards | Quizlet Match each species to its correct oxidation number. - O2 - Na in NaCl - O in H2O. - Fe in FeCl3 - O2 = 0 - Na in NaCl = +1 ... Select all the statements that correctly describe a state function. ... The reaction below has an enthalpy change equal to Q. Match the modified reactions below with their corresponding enthalpies in terms of Q.-Q 1/2Q ...
Label each element in the chemical reaction below with the correct oxidation state.. IMG_1259.jpg - Label each element in the chemical reaction below with ... IMG_1259.jpg - Label each element in the chemical reaction below with the correct oxidation state. +3 +1 -3 +1 NCB (1) + 3 H20 (7) - NH3 (aq) - 3 HOCH | Course Hero Mercy College of Health Science CHEMISTRY CHEMISTRY 120 write the correct oxidation state (correct number andcharge) for each ... Get the detailed answer: write the correct oxidation state (correct number andcharge) for each element in sodium phosphate. Get the detailed answer: write the correct oxidation state (correct number andcharge) for each element in sodium phosphate 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION → ... batteryuniversity.com › article › bu-403-chargingBU-403: Charging Lead Acid - Battery University In the meantime I read a bit about the state of charge of lead acid batteries (mostly Yuasa material) and concluded that, more or less, I should be fine trying to charge it. I charged the battery through a 2 ohm resistor to limit current to a bit below 0.1 C (oh yeah, and the battery has 7 Ah capacity) with a current of 600 mA initially. Chemistry B: Oxidation Reduction Flashcards | Quizlet a number that indicates how many electrons an atom has gained, lost, or shared to form a chemical bond with one or more atoms; in a neutral compound, the sum of oxidation numbers is zero. oxidation-reduction reaction. a reaction in which electrons are transferred from one substance to another substance. oxidizing agent.
Label each element with an oxidation number? | Socratic The oxidation number of chlorine is usually -1. Equation 1 0 Mg + 2+1 H-1 Cl → 0 H2 + +2 Mg-1 Cl2 Listing the atoms in order, the oxidation numbers are: Mg = 0ll (Rule 1) H = +1ll (Rule 5) Cl = -1m (Rules 2 and 6) H = 0ml (Rule 1) Mg = +2 (Rule 3) Cl = -1m (Rules 2 and 6) Equation 2 4 0 Fe + 30 O2 → 2+3 Fe2-2 O3 Oxidation State - Definition, Lowest and Highest Oxidation State ... Each atom in an element either in its free or uncombined state holds up an oxidation number of zero. Clearly, each atom in H 2, Cl 2, P 4, Na, Al, O 2, O 3, S 8, and Mg, has an oxidation number zero. The oxidation number of ions which comprise of only one atom is equal to the actual charge on the ion. Label each element in the chemical reaction below with the correct ... In each of the following equations, indicate the element thathas been oxidized and the one that has been reduced. You shouldalso label the oxidation state of each before and after theprocess: 1) 2 Na +FeCl 2 Chem 111 - Chapter 14 Study Flashcards | Quizlet Redox reaction = a reaction in which electrons are transferred from one species to another Select all the equations that include the oxidation of iron (free element, ion, or in compound) Fe(s) + 2AgNO3(aq) ==> Fe(NO3)2(aq) + 2Ag(s) 2FeBr2(s) + Br2(l) ==> 2FeBr3(s) 4Fe(s) + 3O2(g) ==> 2Fe2O3(s)
› medicine-finder › ozempic-1-mgOzempic 1 mg - NPS MedicineWise Steady-state exposure was achieved following 4-5 weeks of once weekly administration. In patients with type 2 diabetes, the mean steady state concentrations following s.c. administration of 0.5 mg and 1 mg semaglutide were approximately 16 nanomol/L and 30 nanomol/L, respectively. Label each element in the chemical reaction below with the correct ... Nitrogen in both Ammonia and Nitrogen trichloride has an oxidation state of -3. It shares three electrons with each of the three atoms of hydrogen and chlorine. Hydrogen in both Oxochlorate (I) acid and in water has an oxidation state of +1. Each atom shares an election with oxyen in both compounds. Balance and label the oxidation state of each element in each ... - Wyzant Balance and label the oxidation state of each element in each reaction. Log in Sign up. Find A Tutor . Search For Tutors. ... Balance and label the oxidation state of each element in each reaction. Oxidation: Zn --> Zn2+ + 2e-Reduction: As2O3 + 12e- + 6H+ --> 2As3- + 3H2O. Chem chapter 10 Practice Flashcards | Quizlet Match each species to its correct oxidation number. - O2 - Na in NaCl - O in H2O. - Fe in FeCl3 - O2 = 0 - Na in NaCl = +1 ... Select all the statements that correctly describe a state function. ... The reaction below has an enthalpy change equal to Q. Match the modified reactions below with their corresponding enthalpies in terms of Q.-Q 1/2Q ...
› publication › 241210010(PDF) Handbook of Monochromatic XPS Spectra - ResearchGate Jan 01, 1999 · Chemical Abstract Services number, common name, and the Latin language name of the element if available. The second page of each set is the wide scan survey spectrum with peak labels for each of ...
Redox Reactions - Examples, Types, Applications, Balancing - BYJUS The oxidation and reduction reactions always occur simultaneously in redox or Oxidation-Reduction reactions. The substance getting reduced in a chemical reaction is known as the oxidizing agent, while a substance that is getting oxidized is known as the reducing agent. JEE Main 2021 LIVE Chemistry Paper Solutions 24-Feb Shift-1 Memory-Based

0 Response to "44 label each element in the chemical reaction below with the correct oxidation state."
Post a Comment