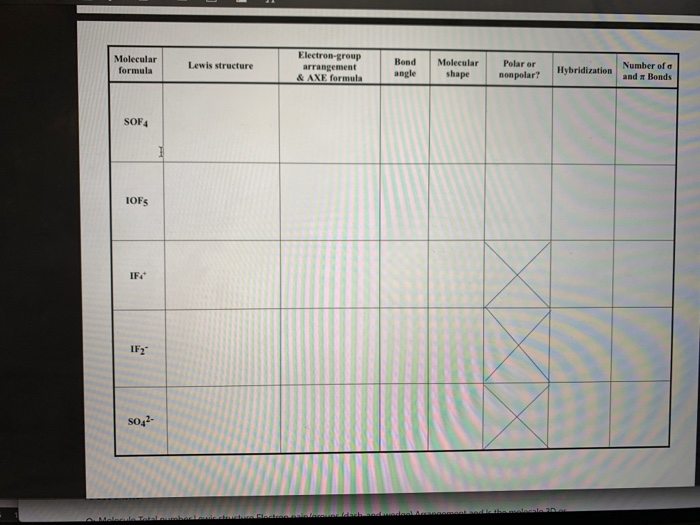
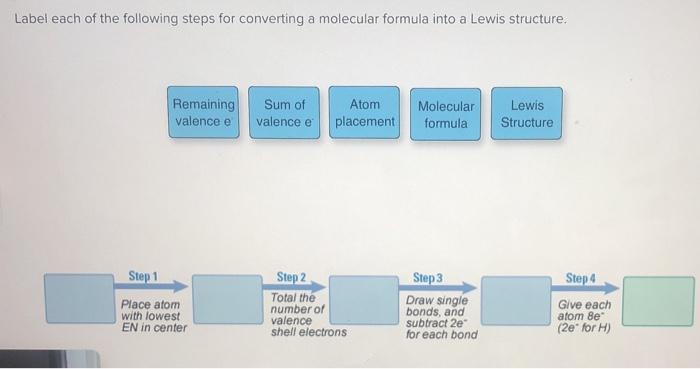
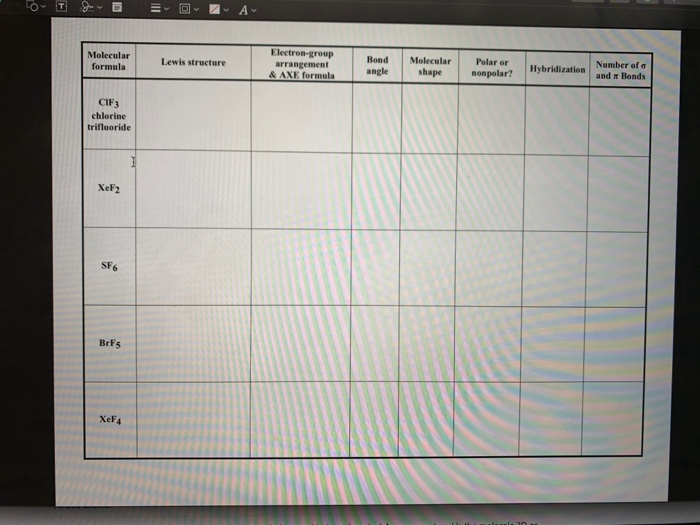
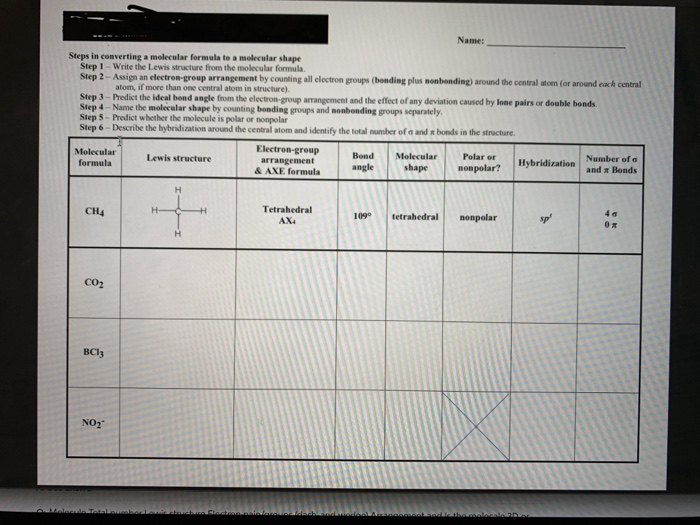
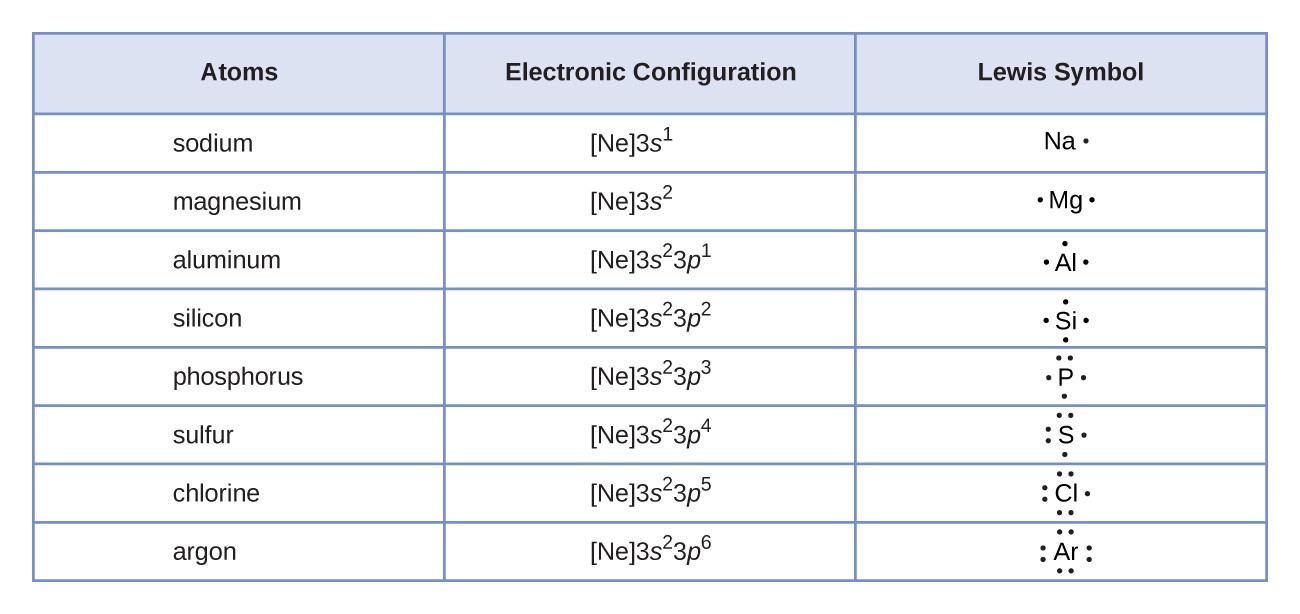
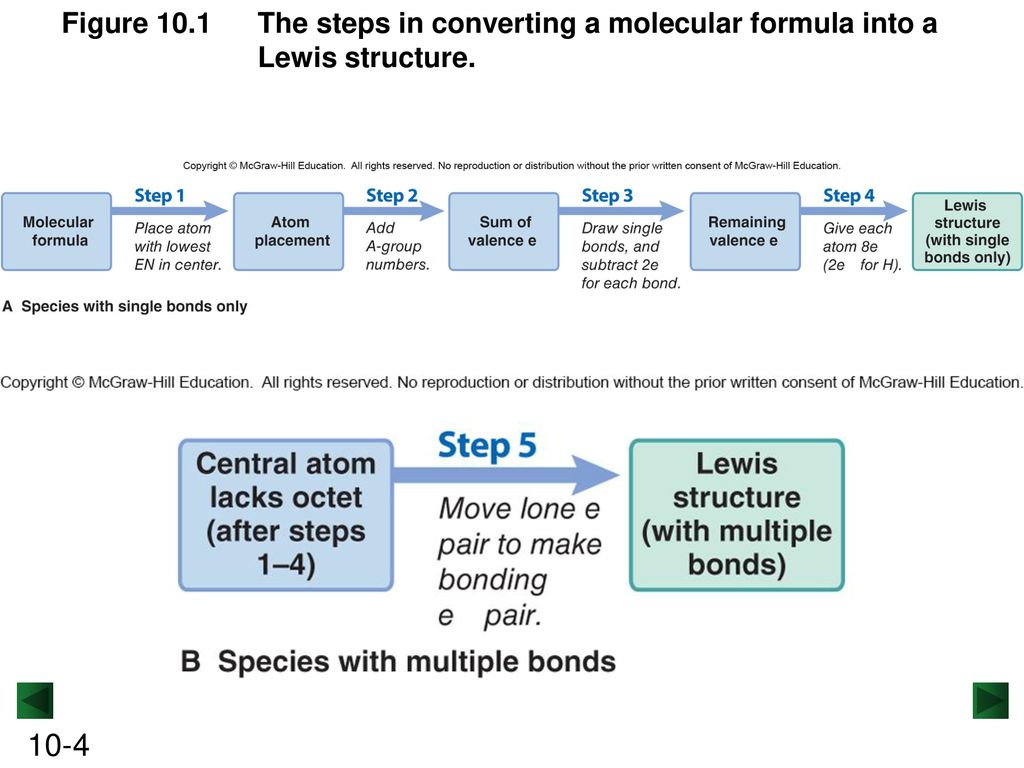
44 label each of the following steps for converting a molecular formula into a lewis structure
Empty string - Wikipedia However, a string comparison function would indicate that all of these empty strings are equal to each other. Even a string of length zero can require memory to store it, depending on the format being used. In most programming languages, the empty string is distinct from a null reference (or null pointer) because a null reference points to no string at all, not even the empty string. The … Solved Label each of the following steps for converting a - Chegg Molecular Remainin Structureformula g valence Lewis Sum of Atom valence eplacement Step 1 Step 2 Step 3 Step 4 Place atom with lowest EN in center Total the number of valence shell electrons Draw single bonds, and subtract 2e for each bond Give each atom 8e (2e for This problem has been solved! See the answer Show transcribed image text
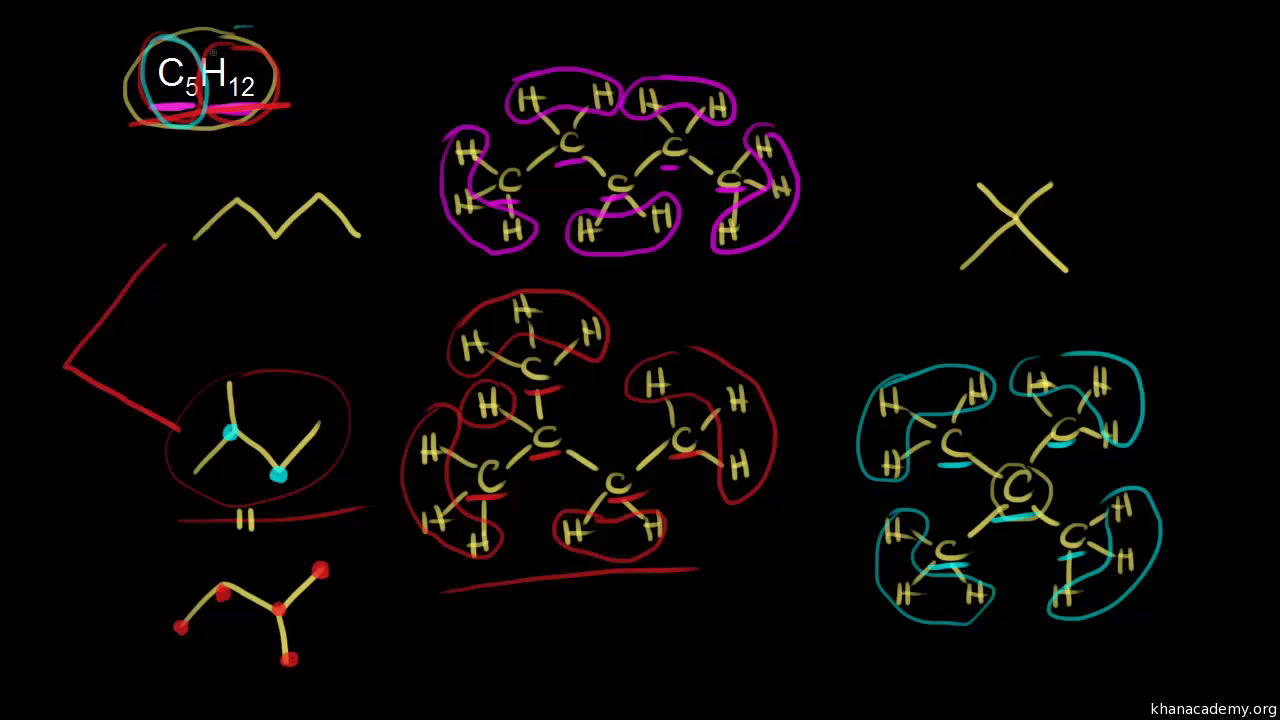
Organic Chemistry 331- Sapling Learning CH 3 Flashcards - Quizlet Choose the correct name for the given structure. Draw the structure of cyclopentane. Draw the structure of 1,3-dimethylcyclohexane. Three structural isomers have the formula C5H12. Draw and name the isomers using IUPAC names. Use a hyphen (-) where necessary, not an en-dash (-).
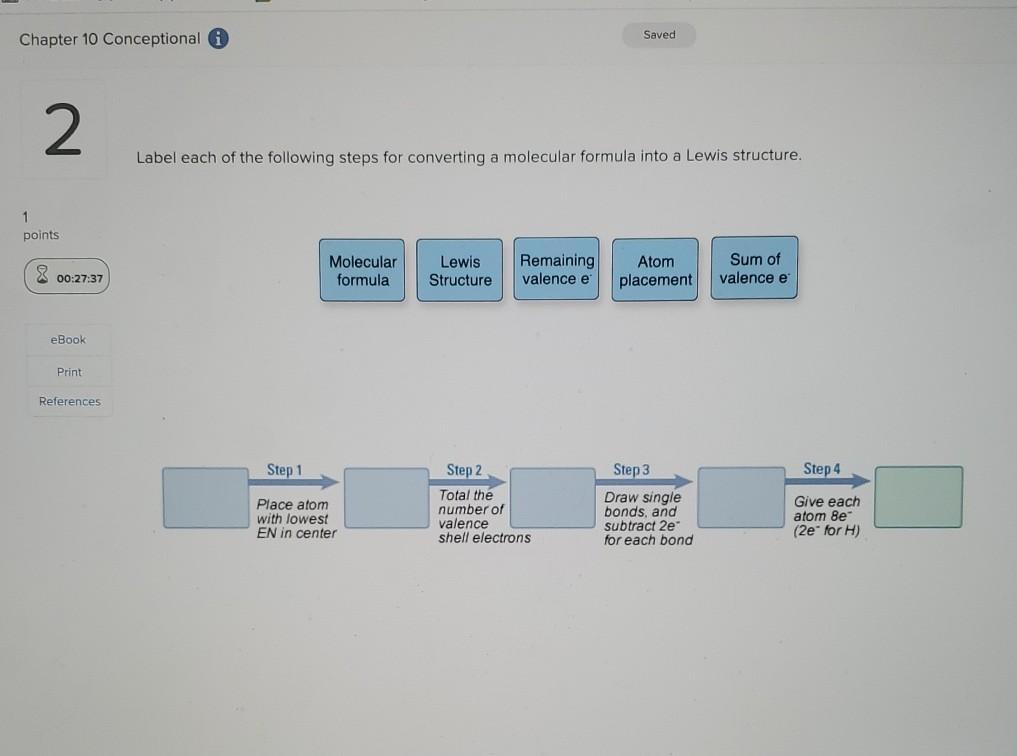
Label each of the following steps for converting a molecular formula into a lewis structure
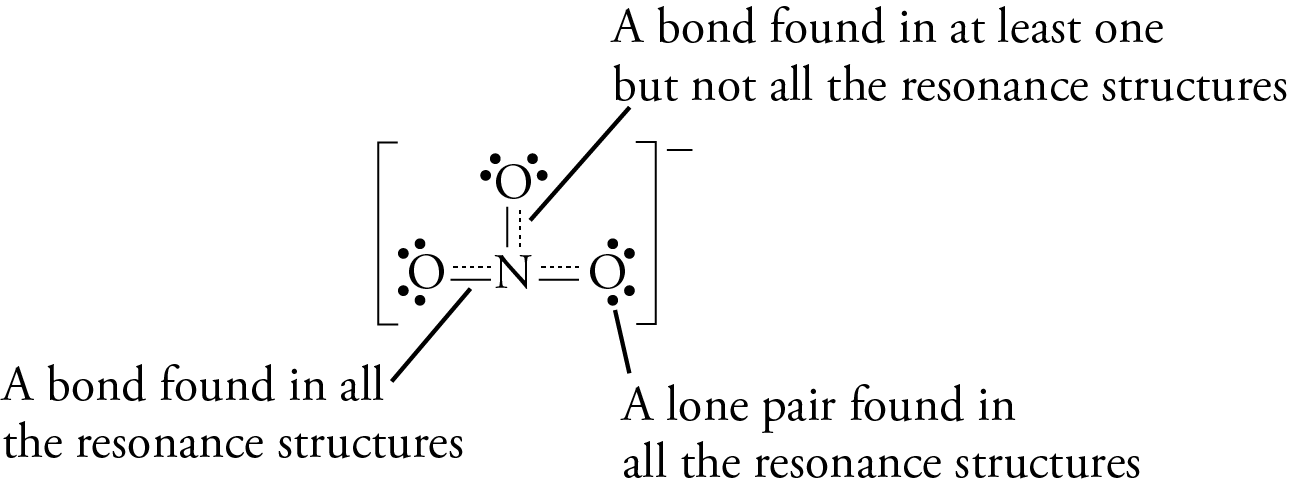
Mastering Chemistry Set Flashcards | Quizlet One of the steps in the commercial process for converting ammonia to nitric acid is the conversion of NH3 to NO: 4NH3 (g)+5O2 (g)→4NO (g)+6H2O (g) In a certain experiment, 1.30 g of NH3NH3 reacts with 2.38 g of O2. A) Which is the limiting reactant? B) How many grams of NO and of H2O form? A) O2 B) 1.78 g, 1.60 g Lewis Structures and the Shapes of Molecules - Angelo State University Draw a single bond from each terminal atom to the central atom. Each bond uses two valence electrons. Distribute the remaining valence electrons in pairs so that each atom obtains eight electrons (or 2 for H). Place the lone pairs on the terminal atoms first , and place any remaining valence electrons on the central atom. Answered: Convert the following molecular… | bartleby Convert the following molecular formulas into line-bond structures that are consistent with valence rules: C3H8 b) CH5N c) C2H6O (2 possibilities) d) C3H7Br (2 possibilities) e) C2H4O (3 possibilities) f) C3H9N (4 possibilities) ... Name of the 3-D drawing Molecular Formula Lewis Structure ... In the following molecule, label all lone pair ...
Label each of the following steps for converting a molecular formula into a lewis structure. BIO 225 Lecture - Chapter 6 Flashcards | Quizlet - take a sample from the broth and plate onto solid media - repeat (multiple times) the incubation of the broth and plating of samples after the same set time period - incubate all plates and count the colonies that develop - place a small number of cells into a sterile broth - incubate the broth for a set time period How to draw organic molecules - chemguide Draw the most detailed formula that you can fit into the space available. If in doubt, draw a fully displayed formula. You would never lose marks for giving too much detail. Apart from the most trivial cases (for example, burning hydrocarbons), never use a molecular formula. Always show the detail around the important part(s) of a molecule. Integrative analysis of metabolite GWAS illuminates the molecular … 08.09.2022 · Pathway diagram and molecular structure of relevant metabolites, colored by their biochemical groups. The pathway diagram was curated from multiple resources (see ‘Methods’). All solid lines represent a single chemical reaction step. Dotted lines represent a simplification of multiple steps. For simplicity, only a subset of all the reactions each metabolite participates in … Condensed structures (video) | Khan Academy - Let's say we're given the molecular formula C three H eight O, and we're asked to draw a Lewis dot structure. So on the left here is one possible Lewis dot structure that you can draw that has that molecular formula. There are three carbons, one, two, three. There's one oxygen, right here. And if you count up the hydrogens you will get eight.
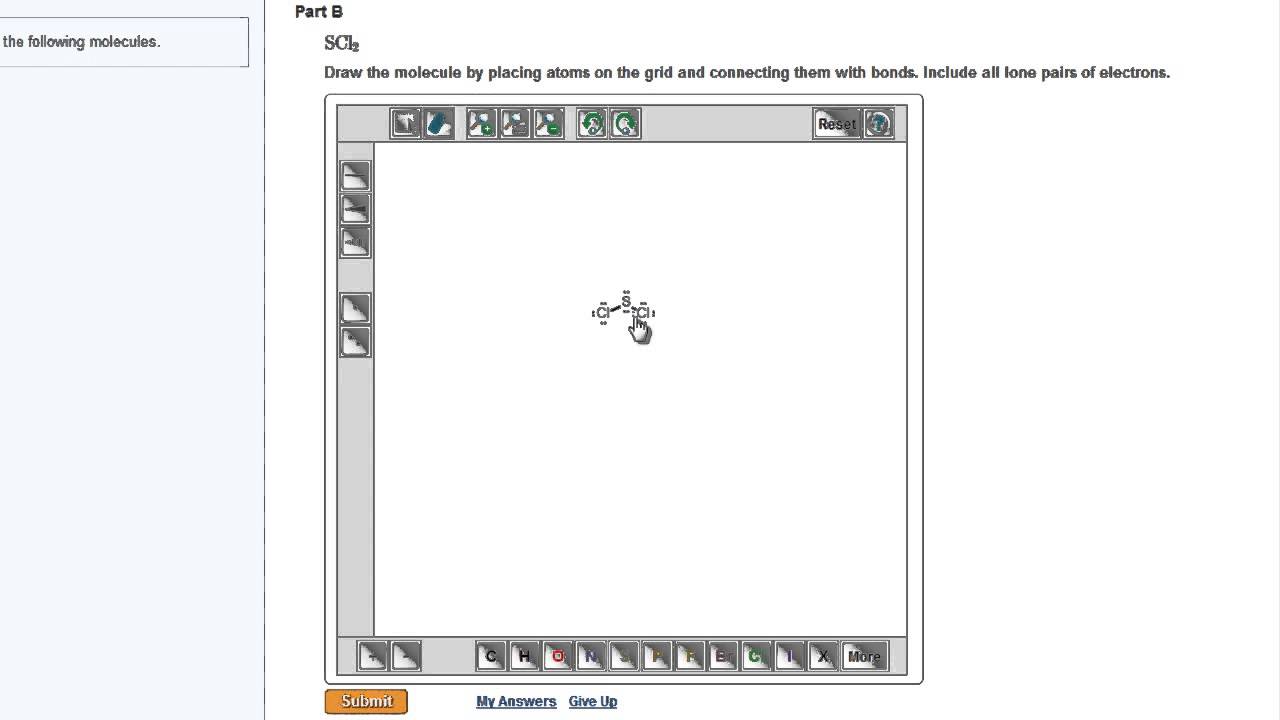
Data analysis - Wikipedia Data analysis is a process of inspecting, cleansing, transforming, and modelling data with the goal of discovering useful information, informing conclusions, and supporting decision-making. Data analysis has multiple facets and approaches, encompassing diverse techniques under a variety of names, and is used in different business, science, and social science domains. How to Draw a Lewis Structure - ThoughtCo Be aware there are several different strategies for constructing Lewis structures. These instructions outline the Kelter strategy to draw Lewis structures for molecules. Step 1: Find the Total Number of Valence Electrons In this step, add up the total number of valence electrons from all the atoms in the molecule. Bond-line, Lewis and Condensed Structures with ... - Chemistry Steps 4) Draw the carbon chain in a zig-zag form. Putting the first atom up or down doesn't matter as long as you keep the correct connectivity of atoms. 5) Erase the carbon atoms together with the hydrogens on them. Keep all the heteroatoms (any atom except carbon) together with the hydrogens on them. Solved Label each of the following steps for converting a | Chegg.com Question: Label each of the following steps for converting a molecular formula into a Lewis structure. Draw single bonds. Subtract 2e for each bond. Place atom with lowest electronegativity in the center. Determine the total number of valence shell electrons. Give each atom 8 e (2 e for H).
Food Chemistry 4th Edition by Belitz, W. Grosch, P. Schieberle … Enter the email address you signed up with and we'll email you a reset link. Molecular Formulas | Introduction to Chemistry | | Course Hero In this case, the empirical formula of glucose is CH 2 O. To convert between empirical and molecular formulas, the empirical formula can be multiplied by a whole number to reach the molecular formula. In this case, the empirical formula would be multiplied by 6 to get to the molecular formula. Examples of Empirical and Molecular Formulas: PDF Drawing Lewis Structures Atoms - preparatorychemistry.com Drawing Lewis Structures (2) •Step 2:Draw a reasonable skeletal structure, using single bonds to join all the atoms. -Try to arrange the atoms to yield the most typical number of bonds for each atom. -Apply the following guidelines in deciding what element belongs in the center of your structure. chem PROCTOR Flashcards | Quizlet 1.) place atom with lowest electronegativity in the center 2.) determine the total number of Valence Shell Electrons 3.) draw the single bonds and subtract two electrons for each bond 4.) give each atom 8 electrons ( 2 electrons for H) steps for converting a molecular formula into a Lewis Structure false
How to Find Molecular Formula: 13 Steps (with Pictures) - wikiHow Then divide the grams of gas by the moles of gas present to yield molecular weight. Example: 14.42 g / 0.0377 mol = 382.49 g/mol. 4. Add together the atomic weight of all atoms in the empirical formula. Each atom in the empirical formula has its own atomic weight.
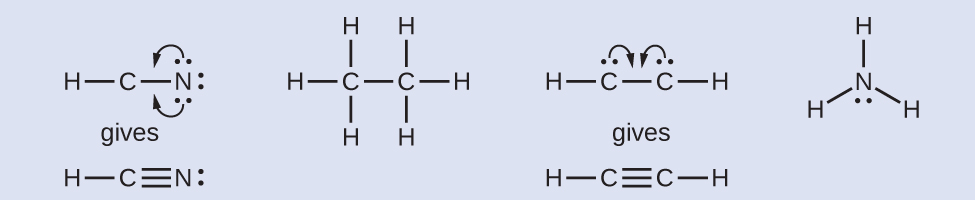
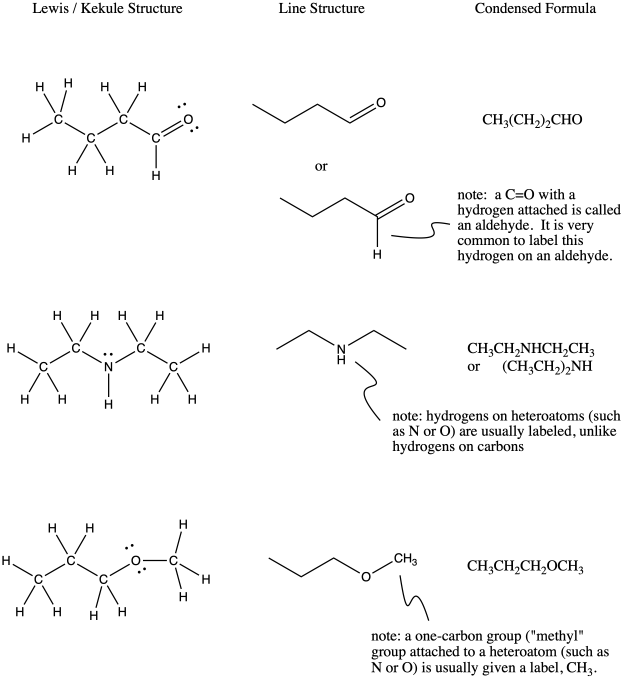
10.1 Condensed Structure and Line Structure - CHEM 1114 - BCcampus Determine the Lewis Structure of the following condensed structure of oleic acid, a fatty acid that is found naturally in various animal and vegetable fats and oils. CH 3 (CH 2) 7 CH=CH (CH 2) 7 COOH Solution Start by drawing the CH3.
Answered: Explain the process of Converting a… | bartleby Solution for Explain the process of Converting a Condensed Structure to a Lewis Structure ?
Answered: Convert the following molecular model… | bartleby Q: Convert the following molecular model into a skeletal structure. ball & stick v + labels A: Skeletal structure of any organic compound is a representation of a series of atoms or groups that… Q: Convert the following condensed formulas into skeletal structures.
How To: Drawing Lewis Structures From Condensed Molecular Formulas There are three steps you should follow to draw a correct structure. 1. From a condensed molecular formula, you obtain information about which atoms are connected to each other in a molecule. Connect all of the appropriate atoms with single bonds first (lines). Example: CH3CH2CH2CO2CH3
Twitpic Dear Twitpic Community - thank you for all the wonderful photos you have taken over the years. We have now placed Twitpic in an archived state.
Solved Label each of the following steps for determining a - Chegg Count all e groupse, place single around central atom (A). nonbonding e first, distribute 8 e to al atoms, ad muliple groups separatelymultple bonds as needed Step2 Molecular Molecular formula Lewis structure Bond angles group Previous question Next question
Lewis Electron Dot Structures - Detailed Explanation with ... - BYJUS The steps that must be followed while drawing a Lewis structure are listed below. First, the total number of valence electrons present in the molecule is calculated by adding the individual valencies of each atom.
Empirical, molecular, and structural formulas - Khan Academy SPQ‑2.A.3 (EK) Transcript. There are three main types of chemical formulas: empirical, molecular and structural. Empirical formulas show the simplest whole-number ratio of atoms in a compound, molecular formulas show the number of each type of atom in a molecule, and structural formulas show how the atoms in a molecule are bonded to each other.
Lewis Structures - chemed.chem.purdue.edu The formula of the compound often provides a hint as to the skeleton structure. The formula for the chlorate ion, for example, suggests the following skeleton structure. The third step assumes that the skeleton structure of the molecule is held together by covalent bonds.
CH2O lewis structure, molecular geometry, bond angle, hybridization? Formaldehyde (CH2O) lewis dot structure, molecular geometry, polar or non-polar, hybridization. Formaldehyde is an organic compound that appears as a colorless gas with the chemical formula CH2O. It is the simplest aldehyde made up of two hydrogens, one carbon, and one oxygen. It is widely used as a preservative because of its antibacterial ...
Converting Bond-Line, Newman, and Fischer Projection - Chemistry Steps And now we need to convert this into the more stable staggered conformation shown in zig-zag. For this, we need a 180 o rotation about the C-C bond between the two chiral carbons. This puts the methyl group (on the rightmost) up and the OH with the Br pointing down changing their wedge and dash notation:
CCL4 Molecular Geometry, Lewis Structure, Hybridization, And Everything For the Lewis structure of CCl4 first, let's calculate the total valence electrons. Carbon has four valence electrons and each Chlorine atom has seven valence electrons. As there are four molecules of Chlorine, we will calculate the number of valence electrons accordingly. = 4 + (4*7) = 4 + 28. = 32 valence electrons.
study set 1 Flashcards | Quizlet A hydrocarbon in which all the bonds between carbon atoms are single bonds. A ring structure is not a. saturated hydrocarbon because two of the hydrogens would have to be removed in the above structure, i.e., CH3CJ2CH2CH3. Alkanes burn=. ___CH3CH3 + ____ O2 ---->____CO2 + ____H2O. b/c they burn, lab based transformation alkanes are not very ...
How to Determine the R and S configuration - Chemistry Steps Step 1: Give each atom connected to the chiral center a priority based on its atomic number. The higher the atomic number, the higher the priority. So, based on this, bromine gets priority one, the oxygen gets priority two, the methyl carbon is the third and the hydrogen is the lowest priority-four: Step 2:
Answered: Convert the following molecular… | bartleby Convert the following molecular formulas into line-bond structures that are consistent with valence rules: C3H8 b) CH5N c) C2H6O (2 possibilities) d) C3H7Br (2 possibilities) e) C2H4O (3 possibilities) f) C3H9N (4 possibilities) ... Name of the 3-D drawing Molecular Formula Lewis Structure ... In the following molecule, label all lone pair ...
Lewis Structures and the Shapes of Molecules - Angelo State University Draw a single bond from each terminal atom to the central atom. Each bond uses two valence electrons. Distribute the remaining valence electrons in pairs so that each atom obtains eight electrons (or 2 for H). Place the lone pairs on the terminal atoms first , and place any remaining valence electrons on the central atom.
Mastering Chemistry Set Flashcards | Quizlet One of the steps in the commercial process for converting ammonia to nitric acid is the conversion of NH3 to NO: 4NH3 (g)+5O2 (g)→4NO (g)+6H2O (g) In a certain experiment, 1.30 g of NH3NH3 reacts with 2.38 g of O2. A) Which is the limiting reactant? B) How many grams of NO and of H2O form? A) O2 B) 1.78 g, 1.60 g
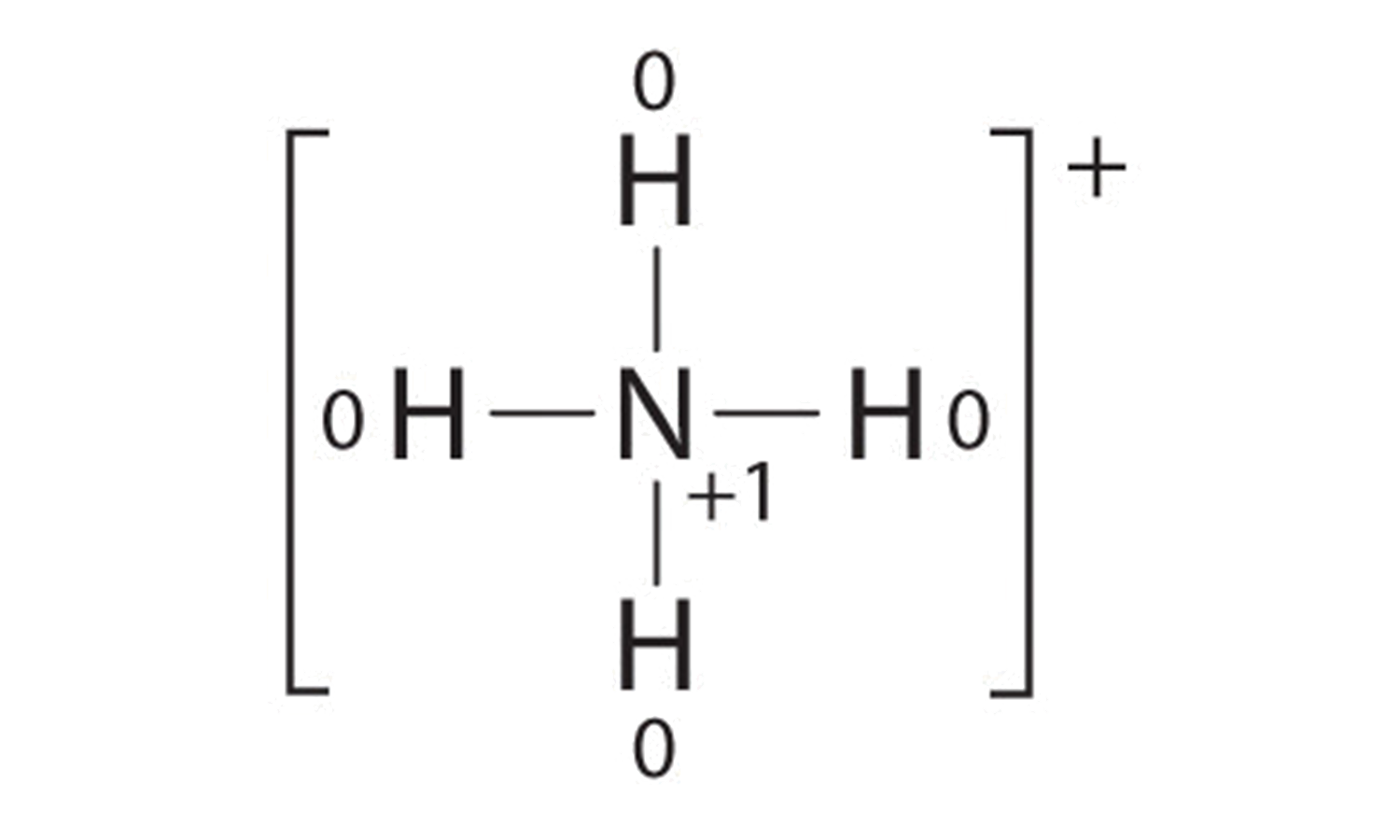




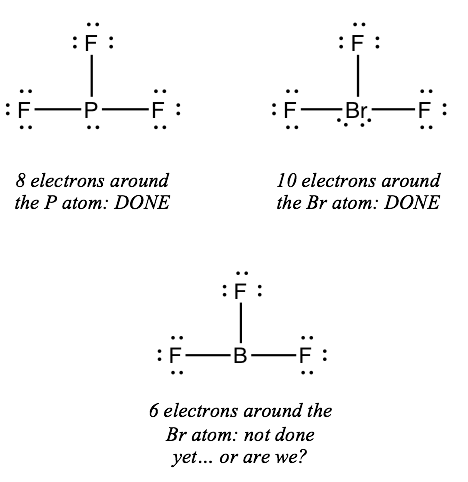


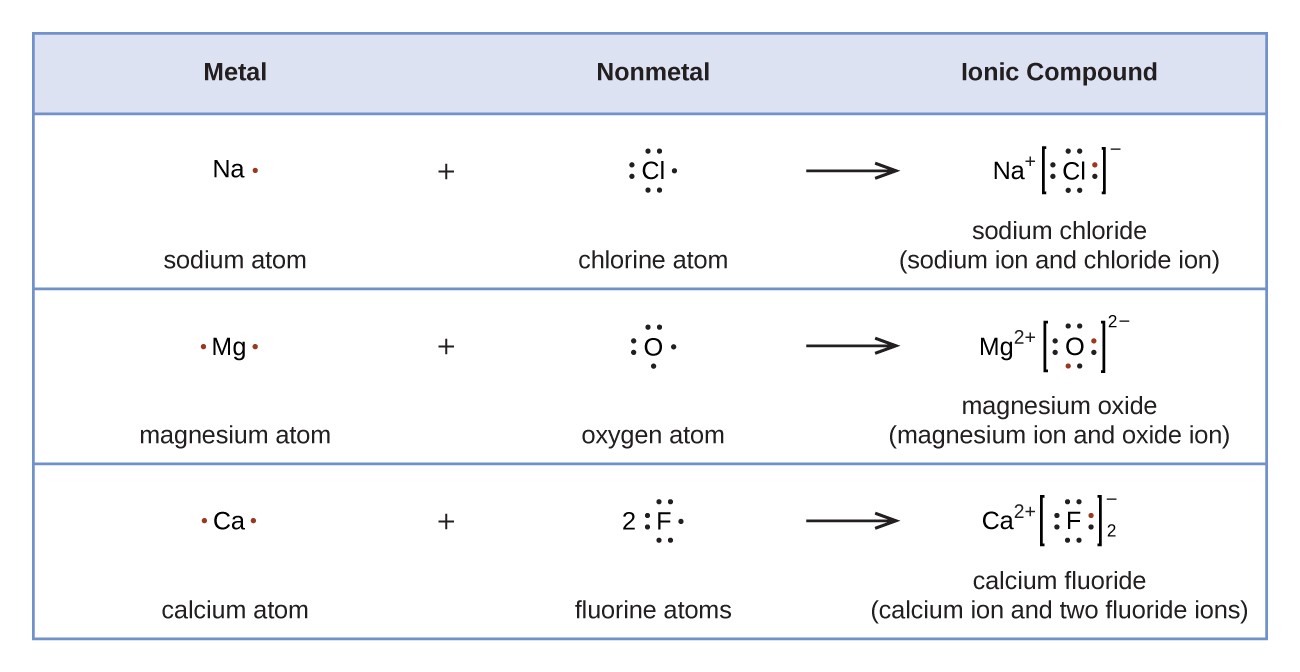
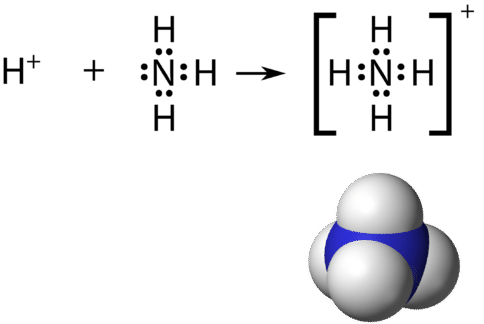
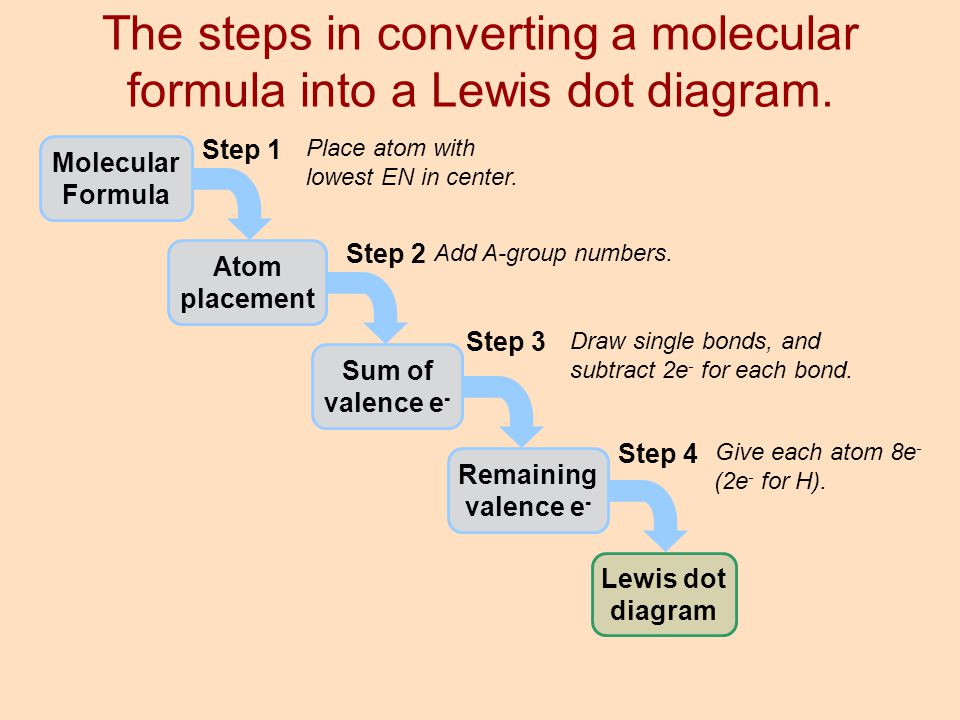
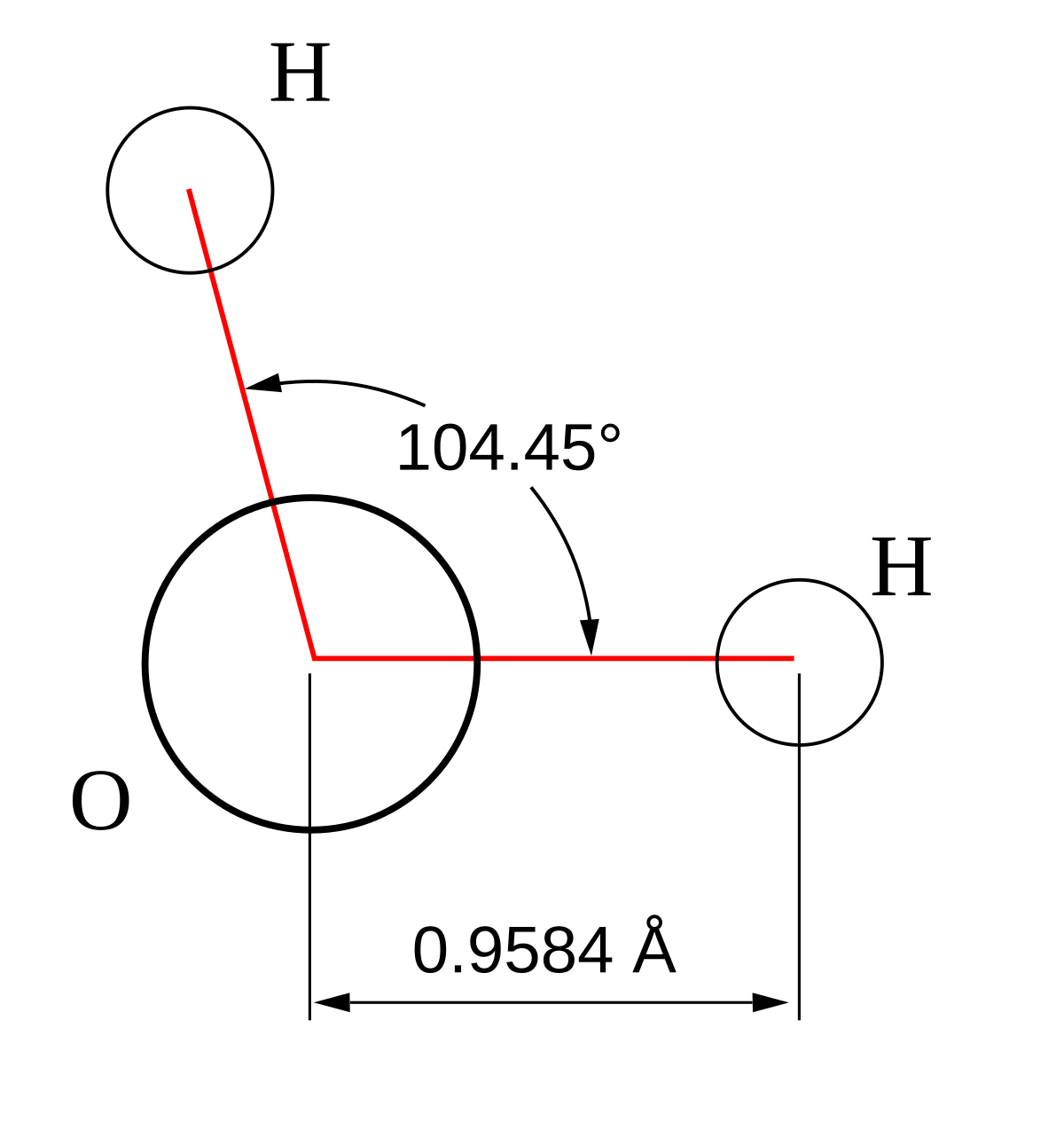











:max_bytes(150000):strip_icc()/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)

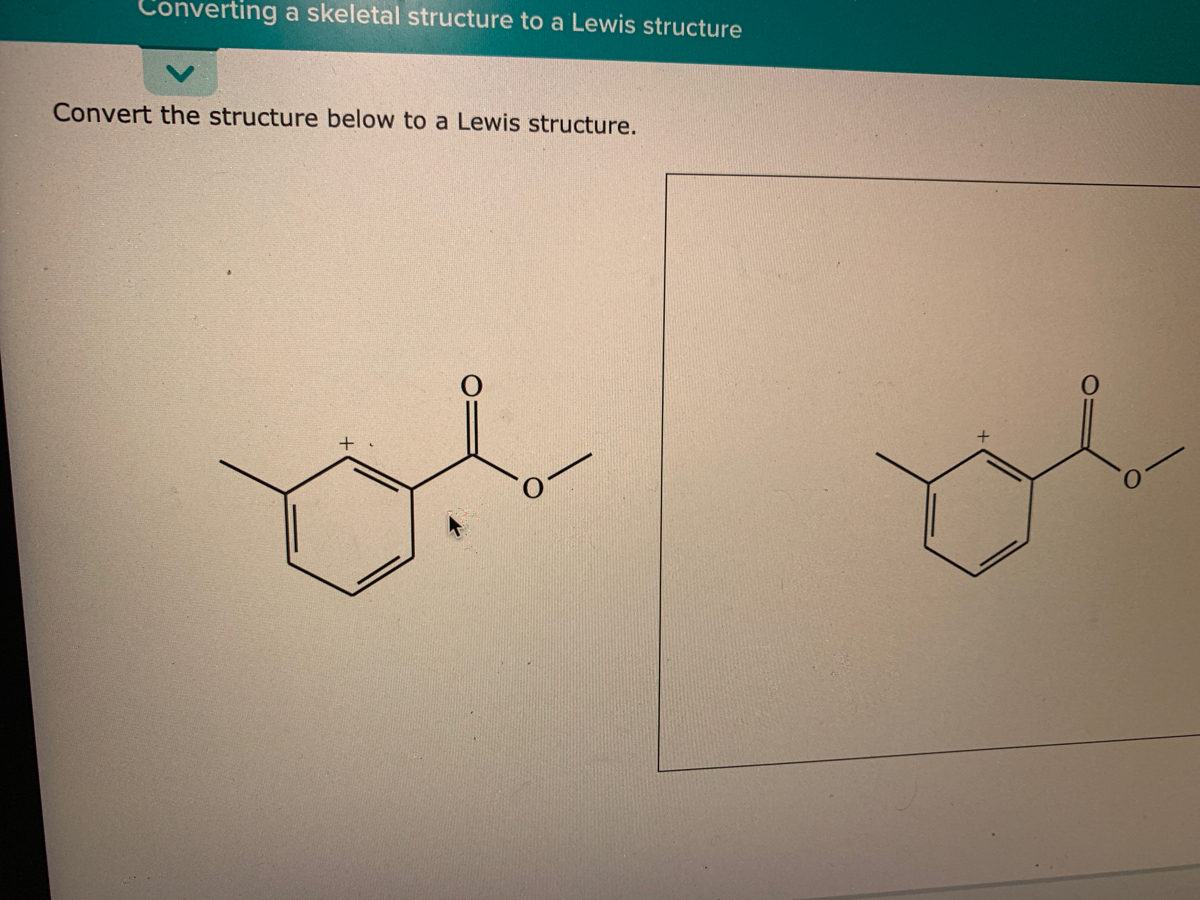
0 Response to "44 label each of the following steps for converting a molecular formula into a lewis structure"
Post a Comment