38 off label use of medical devices
Off-Label Use: Patient Safety Implications | The Doctors Company The FDA defines “off-label” use as the unapproved use of an approved medication or product. The approved medication is used for an indication, dosage amount or ... Off-label use of medicinal products - European Union patients, and the regulatory framework for the off-label use of medicines. ... medicinal products for veterinary use and medical devices.
Off-label use of a medical device - GOV.UK Dec 18, 2014 ... If you use the device in any other way, it's considered 'off-label' use. Without the manufacturer's approval this will be at your own risk and ...

Off label use of medical devices
FDA to clarify role of off-label uses in medical device approvals Sep 23, 2020 ... The FDA has released proposed regulations to make clear that off-label use of a device alone will not be enough to sway the agency to give ... MHRA on Off-Label Use of Medical Devices - RegDesk Feb 11, 2021 ... Under the general rule, any medical device should be used strictly in accordance with the instructions for use initially provided by the ... Expanded Access for Medical Devices | FDA - U.S. Food and ... Jun 21, 2019 · Expanded access is a potential pathway for patients with a serious or life-threatening disease or condition to access an investigational medical device that has not been approved or cleared by the ...
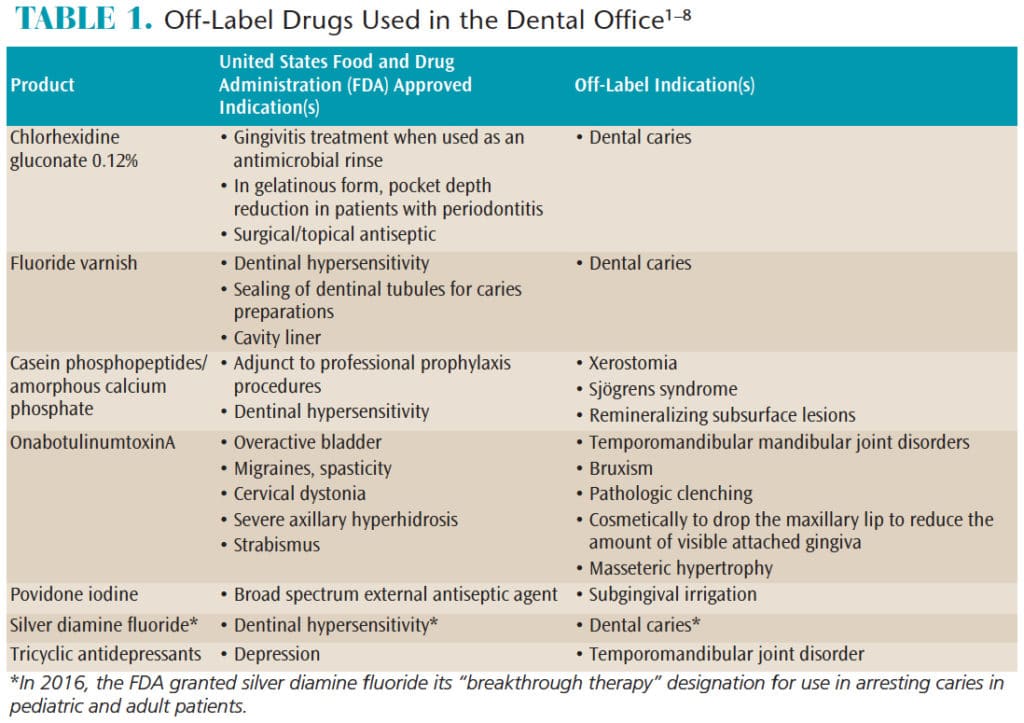
Off label use of medical devices. Medical devices: off-label use - GOV.UK Dec 18, 2014 ... It's considered off-label use if you use a medical device differently to how the manufacturer has instructed. This includes changing a ... Issues associated with off label use of medical devices - PubMed Off label use as applied to medical devices is the application of the device for a purpose that is not included as an indication in the FDA and EC approved ... Understanding Unapproved Use of Approved Drugs "Off Label" Used for a disease or medical condition that it is not approved to treat, such as when a chemotherapy is approved to treat one type of cancer, but healthcare providers use it to treat a different ... Responding to Unsolicited Requests for Off-Label Information ... Responding to Unsolicited Requests for Off-Label Information About Prescription Drugs and Medical Devices December 2011 Download the Draft Guidance Document Read the Federal Register Notice Draft
"Off-Label" and Investigational Use Of Marketed Drugs ... May 06, 2020 · Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices according to their best knowledge and judgement. Off-label use - Wikipedia Off-label use is the use of pharmaceutical drugs for an unapproved indication or in an unapproved age group, dosage, or route of administration. Both prescription drugs and over-the-counter drugs (OTCs) can be used in off-label ways, although most studies of off-label use focus on prescription drugs. UpToDate {{configCtrl2.info.metaDescription}} Sign up today to receive the latest news and updates from UpToDate. Sign Up Off-label use of medical devices: Frequently asked questions The Therapeutic Goods Act 1989 does not regulate clinical practice. 'off-label use' is a clinical decision made at the discretion of the treating clinician who is responsible for obtaining informed consent from their patient and ensuring that the medical device is the appropriate treatment option and carries a positive benefit–risk profile.
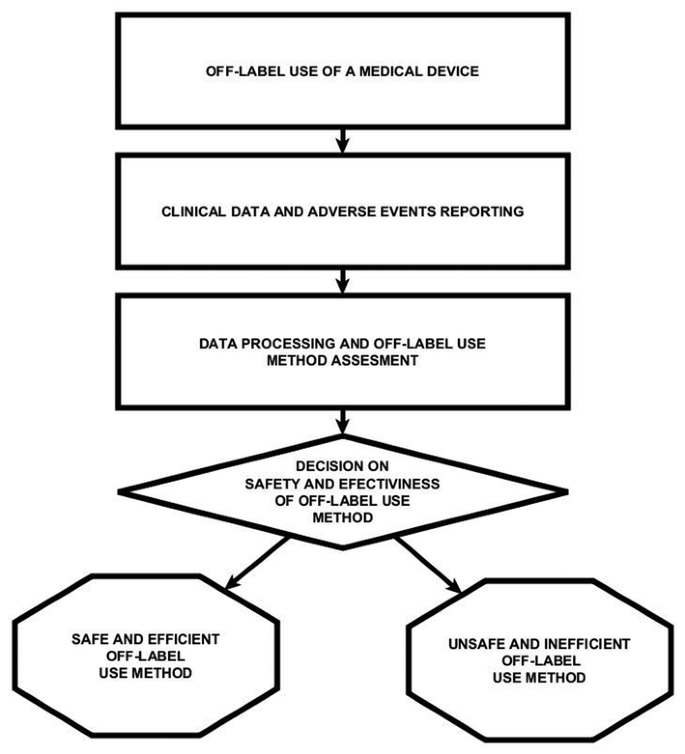
Off-label Use of Medical Devices May 21, 2017 ... Off-label Use of Medical Devices ... Any information that comes with a product is considered labelling and when the product is used for a clinical ... Expanded Access for Medical Devices | FDA - U.S. Food and ... Jun 21, 2019 · Expanded access is a potential pathway for patients with a serious or life-threatening disease or condition to access an investigational medical device that has not been approved or cleared by the ... MHRA on Off-Label Use of Medical Devices - RegDesk Feb 11, 2021 ... Under the general rule, any medical device should be used strictly in accordance with the instructions for use initially provided by the ... FDA to clarify role of off-label uses in medical device approvals Sep 23, 2020 ... The FDA has released proposed regulations to make clear that off-label use of a device alone will not be enough to sway the agency to give ...




























0 Response to "38 off label use of medical devices"
Post a Comment