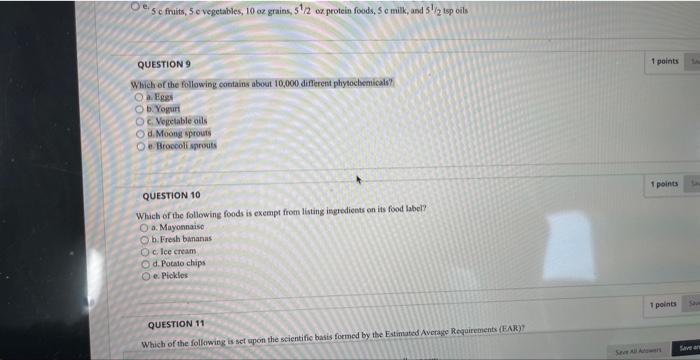
38 which of the following foods is exempt from listing ingredients on the label
National Bioengineered Food Disclosure Standard - Federal … 21/12/2018 · The following foods comprise the List of ... AMS agrees that if they qualify as incidental additives that are not required to be labeled as ingredients on a food label, then they do not require disclosure as BE foods. However, bioengineered yeasts, enzymes, and other organisms that do not qualify as incidental additives that are not required to be labeled as … › current › title-21eCFR :: 21 CFR Part 73 -- Listing of Color Additives Exempt ... (2) The label of food products intended for human use, including butter, cheese, and ice cream, that contain cochineal extract or carmine shall specifically declare the presence of the color additive by listing its respective common or usual name, “cochineal extract” or “carmine,” in the statement of ingredients in accordance with ...
› fintech › cfpb-funding-fintechU.S. appeals court says CFPB funding is unconstitutional ... Oct 20, 2022 · That means the impact could spread far beyond the agency’s payday lending rule. "The holding will call into question many other regulations that protect consumers with respect to credit cards, bank accounts, mortgage loans, debt collection, credit reports, and identity theft," tweeted Chris Peterson, a former enforcement attorney at the CFPB who is now a law professor at the University of Utah.

Which of the following foods is exempt from listing ingredients on the label
Food coloring - Wikipedia Food coloring, or color additive, is any dye, pigment, or substance that imparts color when it is added to food or drink.They come in many forms consisting of liquids, powders, gels, and pastes.Food coloring is used in both commercial food production and domestic cooking. Food colorants are also used in a variety of non-food applications, including cosmetics, … › current › title-21eCFR :: 21 CFR Part 1 -- General Enforcement Regulations (14) The unit containers in a multiunit or multicomponent retail food package shall be exempt from regulations of section 403 (e)(1), (g)(2), (i)(2), (k), and (q) of the act with respect to the requirements for label declaration of the name and place of business of the manufacturer, packer, or distributor; label declaration of ingredients; and ... U.S. appeals court says CFPB funding is unconstitutional - Protocol 20/10/2022 · That means the impact could spread far beyond the agency’s payday lending rule. "The holding will call into question many other regulations that protect consumers with respect to credit cards, bank accounts, mortgage loans, debt collection, credit reports, and identity theft," tweeted Chris Peterson, a former enforcement attorney at the CFPB who is now a law …
Which of the following foods is exempt from listing ingredients on the label. en.wikipedia.org › wiki › Food_coloringFood coloring - Wikipedia The U.S. FDA's permitted colors are classified as subject to certification or exempt from certification in Code of Federal Regulations – Title 21 Part 73 & 74 Archived October 23, 2008, at the Wayback Machine, both of which are subject to rigorous safety standards prior to their approval and listing for use in foods. TBT - European Commission 27/10/2022 · European Union - 2022/11/10 Draft Commission Implementing Regulation amending Regulation EC No 28702000 laying down Community reference methods for the analysis of spirit drinks, and repealing Regulation EEC No 200992 determining Community analysis methods for ethyl alcohol of agricultural origin in the preparation of spirit drinks, aromatized wines, … eCFR :: 21 CFR Part 1 -- General Enforcement Regulations The following exemptions are granted from label statements required by this part: (a) Foods. (1) While held for sale, a food shall be exempt from the required declaration of net quantity of contents specified in this part if said food is received in bulk containers at a retail establishment and is accurately weighed, measured, or counted either within the view of the purchaser or in … › scripts › cdrhCFR - Code of Federal Regulations Title 21 - Food and Drug ... Jul 20, 2022 · (ii) In the absence of a reference amount customarily consumed in § 101.12(b) that is appropriate for the variety or assortment of foods in a gift package, the following may be used as the standard serving size for purposes of nutrition labeling of foods subject to this paragraph: 1 ounce for solid foods; 2 fluid ounces for nonbeverage liquids ...
› documents › 2018/12/21Federal Register :: National Bioengineered Food Disclosure ... Dec 21, 2018 · The NBFDS uses the same methods FDA uses to determine predominance at 21 CFR 101.4(a)(1), which provides that ingredients required to be declared on the label or labeling of a food, including foods that comply with standards of identity, except those ingredients exempted by § 101.100, shall be listed by common or usual name in descending order ... Overview of Food Ingredients, Additives & Colors | FDA On a product label, the ingredients are listed in order of predominance, with the ingredients used in the greatest amount first, followed in descending order by those in smaller amounts. The label ... › mdard › food-dairyMDARD - Michigan Cottage Foods Information Do I have to put a label on my Cottage Foods? Yes, you are required to individually label your Cottage Foods prior to sale. The basic information that must be on the label is as follows: Name and physical address of the Cottage Food operation (You must use the physical address of your home kitchen; Post Office Box addresses are not adequate). Lifestyle | Daily Life | News | The Sydney Morning Herald The latest Lifestyle | Daily Life news, tips, opinion and advice from The Sydney Morning Herald covering life and relationships, beauty, fashion, health & wellbeing
News and Insights | Nasdaq 07/10/2022 · Get the latest news and analysis in the stock market today, including national and world stock market news, business news, financial news and more CFR - Code of Federal Regulations Title 21 - Food and Drug … 20/07/2022 · (ii) In the absence of a reference amount customarily consumed in § 101.12(b) that is appropriate for the variety or assortment of foods in a gift package, the following may be used as the standard serving size for purposes of nutrition labeling of foods subject to this paragraph: 1 ounce for solid foods; 2 fluid ounces for nonbeverage liquids (e.g., syrups); 8 ounces for … Microsoft is building an Xbox mobile gaming store to take on … 19/10/2022 · Microsoft’s Activision Blizzard deal is key to the company’s mobile gaming efforts. Microsoft is quietly building a mobile Xbox store that will rely on Activision and King games. U.S. appeals court says CFPB funding is unconstitutional - Protocol 20/10/2022 · That means the impact could spread far beyond the agency’s payday lending rule. "The holding will call into question many other regulations that protect consumers with respect to credit cards, bank accounts, mortgage loans, debt collection, credit reports, and identity theft," tweeted Chris Peterson, a former enforcement attorney at the CFPB who is now a law …
› current › title-21eCFR :: 21 CFR Part 1 -- General Enforcement Regulations (14) The unit containers in a multiunit or multicomponent retail food package shall be exempt from regulations of section 403 (e)(1), (g)(2), (i)(2), (k), and (q) of the act with respect to the requirements for label declaration of the name and place of business of the manufacturer, packer, or distributor; label declaration of ingredients; and ...
Food coloring - Wikipedia Food coloring, or color additive, is any dye, pigment, or substance that imparts color when it is added to food or drink.They come in many forms consisting of liquids, powders, gels, and pastes.Food coloring is used in both commercial food production and domestic cooking. Food colorants are also used in a variety of non-food applications, including cosmetics, …


:max_bytes(150000):strip_icc()/GettyImages-1293046606-aa02ae19c9e04266842d4725768cc0d2.jpg)





























0 Response to "38 which of the following foods is exempt from listing ingredients on the label"
Post a Comment