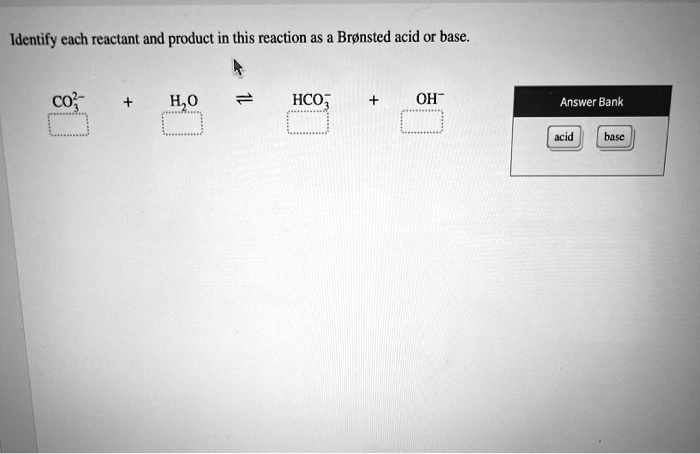
39 label each reactant and product in this reaction as a bronsted acid or base
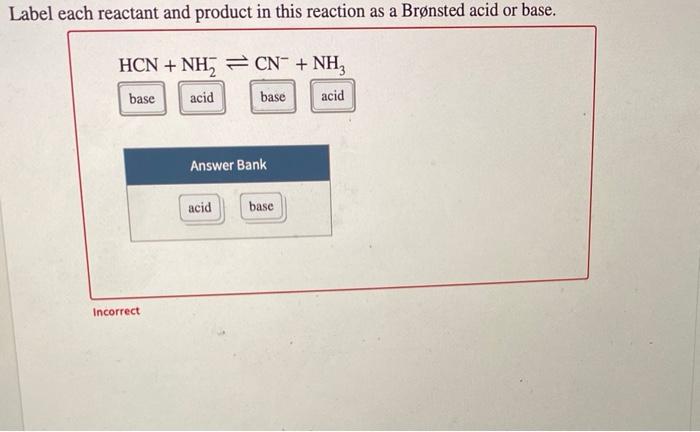
Label each reactant and product in this reaction as a Bronsted acid or ... Label each reactant and product in this reaction as a Brønsted acid or base. HCN + NH 2- >><< CN - + NH 3 harlequinbadger952 In each of the following acid-base reactions, identify the Bronsted acid and base on the left and their conjugate partners on the right 1. HCO2H (aq) + H2O (l) <--------->HCO2- (aq) + H3O+ 2. resources.finalsite.net › images › v1529445793AP Chemistry Scope & Sequence reaction, given the initial amount of one reactant (or product) and assuming the other reactant is in excess. • Determine the limiting reagent for a chemical reaction and the maximum amount of product that can be formed, given the initial amounts of each reactant in the chemical equation. • Calculate percent yield, given the actual amount ...
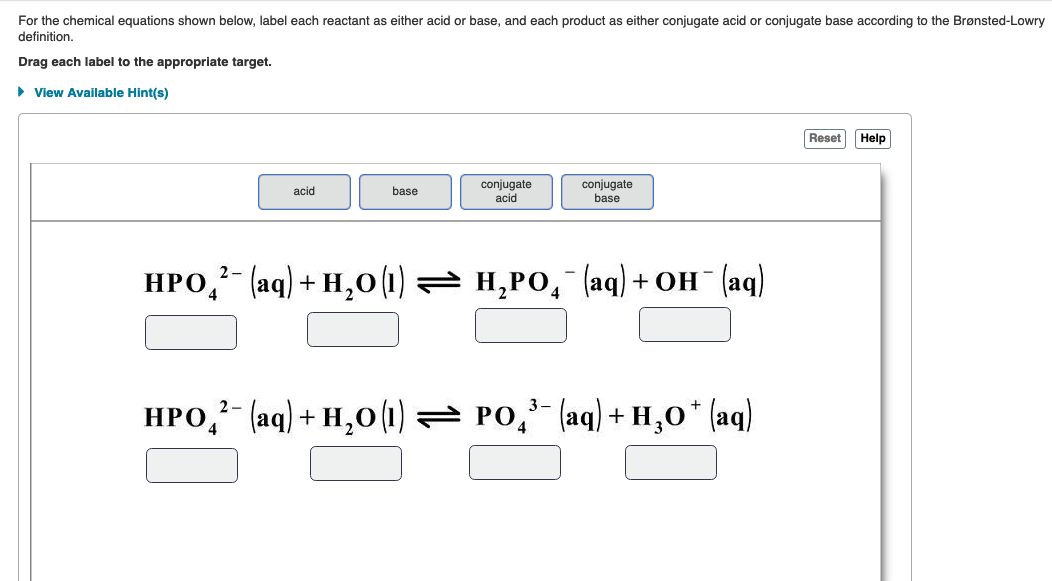
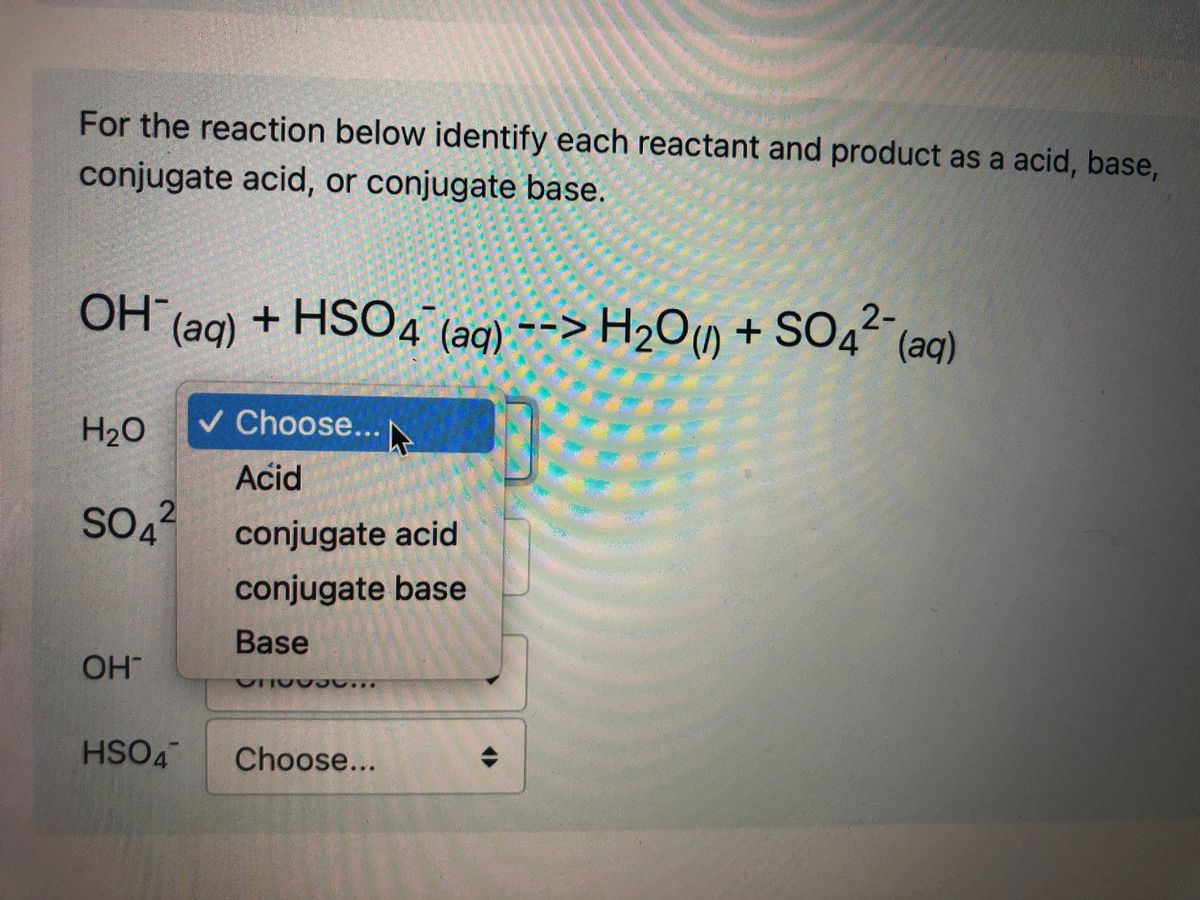
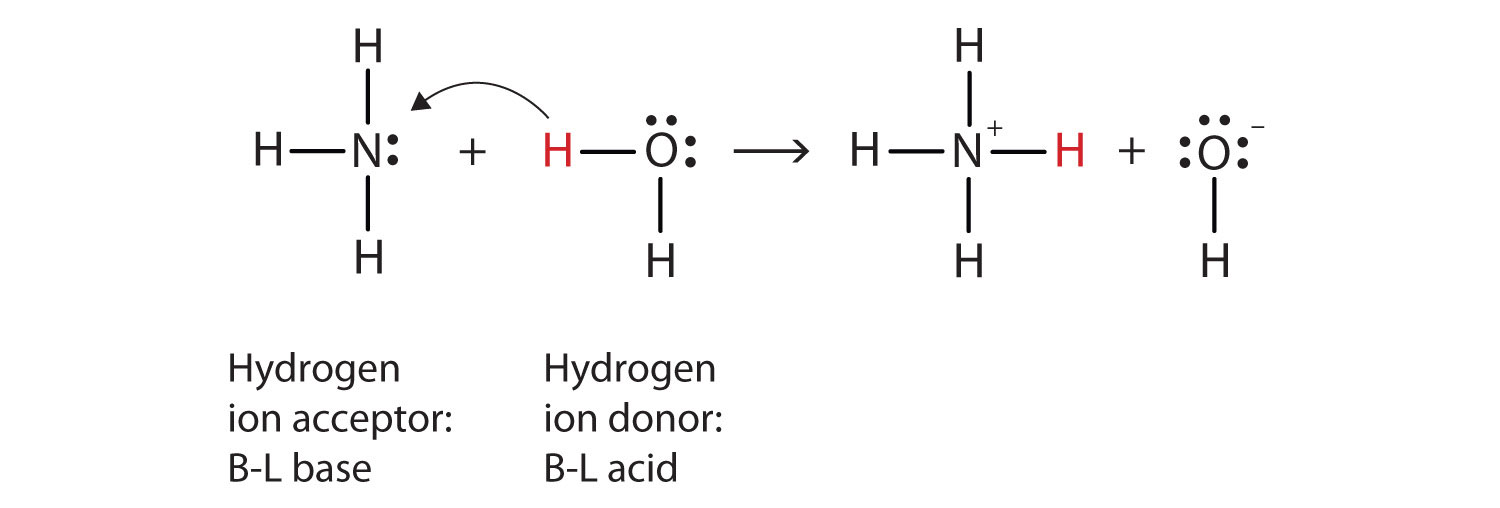
Classify each reactant and product in this reaction as an acid or base ... Explanation: In the Bronsted-Lowry acid-base theory, the acid in a reaction is the species that loses a proton, . The resultant species would be the conjugate base of that acid. On the other hand, the Bronsted-Lowry base in a reaction is the species that accepts a proton . The resultant species would be the conjugate acid of that base.
Label each reactant and product in this reaction as a bronsted acid or base
Label each reactant and product in this reaction as a Brønsted acid or ... Identify each reactant and product in this reaction as a Brønsted acid or base. HY +H,Z = H,Y + HZ- Answer Bank acid base For the given reactions, classify each reactant as a Lewis acid-base, a Brønsted-Lowry acid-base, or both.... For the given reactions, classify each reactant as a Lewis acid-base, a Brønsted-Lowry acid-base, or both. Label each reactant and product in this reaction as a Brønsted acid or ... Get the detailed answer: Label each reactant and product in this reaction as a Brønsted acid or base. HCN + NH2- >><< CN- + NH3. Get the detailed answer: Label each reactant and product in this reaction as a Brønsted acid or base. HCN + NH2- >><< CN- + NH3 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION → ... Label each reactant and product in this reaction as a brønsted acid or ... Classify each of the following reactants and products as an acid or base according to the Bronsted theory CF3COOH + H2,0 H3O+ + CF3COO- Answer General guidance Concepts and reason Classify the given compounds or ions in the reactants and products as Bronsted acids or base based on the whether the group accepts the proton or donate the protons.
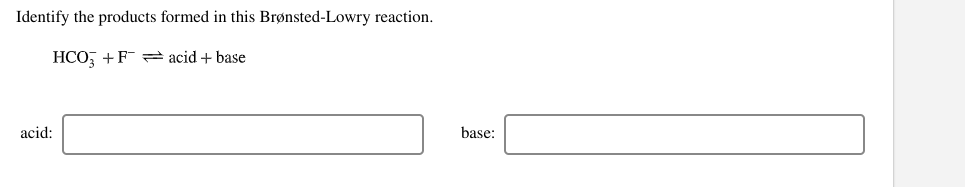
Label each reactant and product in this reaction as a bronsted acid or base. Question : Question label each reactant and product in this reaction as ... Question Label each reactant and product in this reaction as a Bronsted acid or base. Write the balanced chemical equation for the reaction of the weak... Question 1) based on microwave heating which compound would heat most rapidly A) 1-propanol b) 1-bromopropane c) hexane 2) based on microwave heating which compound would... chemical reaction - The Brønsted-Lowry theory | Britannica A somewhat more general acid-base theory, the Brønsted-Lowry theory, named after Danish chemist Johannes Nicolaus Brønsted and English chemist Thomas Martin Lowry, defines an acid as a proton donor and a base as a proton acceptor. In this theory, the reaction of an acid and base is represented as an equilibrium reaction. acid (1) + base (2) ⇌ base (1) + acid (2) (The double arrows, ⇌ ... Label each reactant and product in this reaction as a Bronsted acid or base Label each reactant and product in this reaction as a Bronsted acid or base - YouTube 🚀To book a personalized 1-on-1 tutoring session:👉Janine The Tutorhttps://janinethetutor.com🚀More proven... Label each reactant and product in this reaction as a… - SolvedLib Which of the following statements is true (You can select multiple answers if you think so) Your answer: Actual yield is calculated experimentally and gives an idea about the succeed of an experiment when compared to theoretical yield: In acid base titration experiment; our scope is finding unknown concentration of an acid or base: In the ...
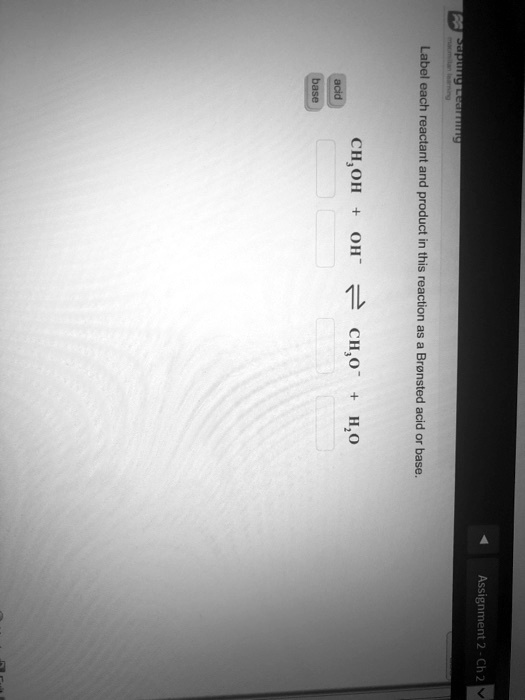
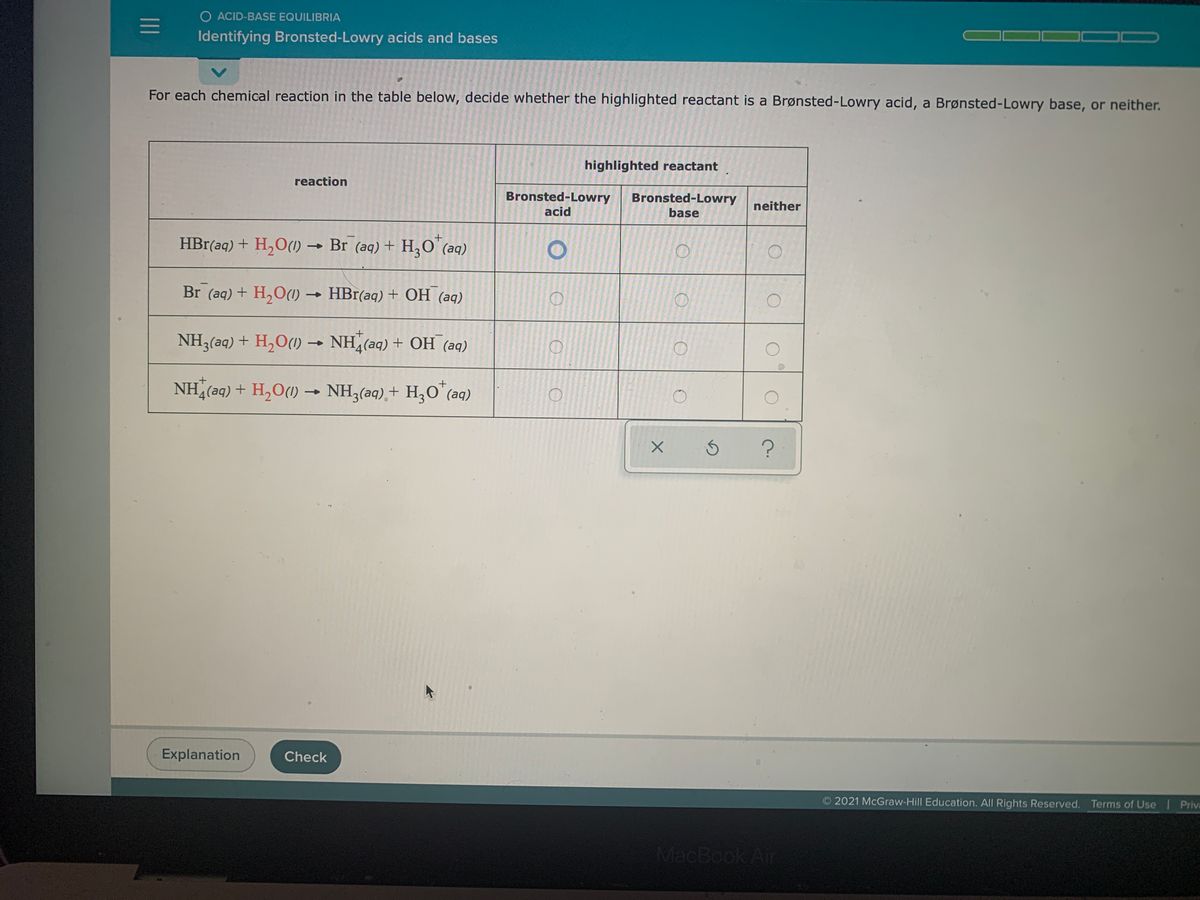
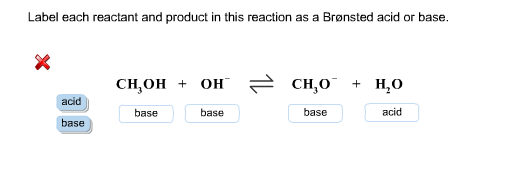
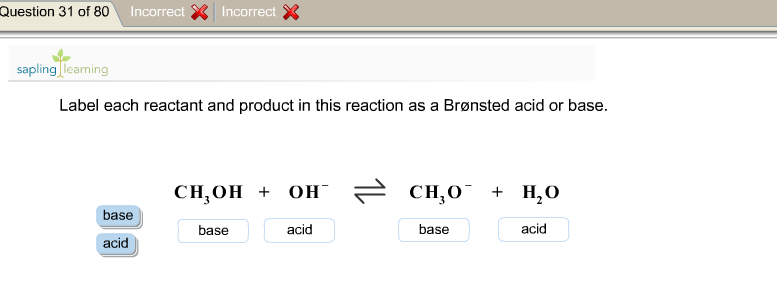
Solved > Question Label each reactant and product in the:996540 ... Question Label each reactant and product in the equilibrium reaction below . Not my Question Bookmark. Flag Content. ... 996540. Question. Label each reactant and product in the equilibrium reaction below as a Bronsted acidor base. HX-2 + W-2 <-----> X-2 + HW. a) base, acid, base, acid. b) acid, base, base, acid ... Label each reactant and product in this reaction as a Brønsted acid or ... Verified answer. Bronstead acids donate H+, bases accept H+. So look to see what happens when you go to the other side of the arrow. CH3OH - acid (it loses an H forming CH30-) OH - base (gains an H forming H2O) CH3O- - base (gains an H forming CH3OH) H2O - acid (loses an H forming OH-) 3 votes Thanks 3. More Questions From This User See All. Label each reactant and product in this reaction as a brønsted acid or ... Classify each of the following reactants and products as an acid or base according to the Bronsted theory CF3COOH + H2,0 H3O+ + CF3COO- Answer General guidance Concepts and reason Classify the given compounds or ions in the reactants and products as Bronsted acids or base based on the whether the group accepts the proton or donate the protons. Label each reactant and product in this reaction as a Brønsted acid or ... Get the detailed answer: Label each reactant and product in this reaction as a Brønsted acid or base. HCN + NH2- >><< CN- + NH3. Get the detailed answer: Label each reactant and product in this reaction as a Brønsted acid or base. HCN + NH2- >><< CN- + NH3 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION → ...
Label each reactant and product in this reaction as a Brønsted acid or ... Identify each reactant and product in this reaction as a Brønsted acid or base. HY +H,Z = H,Y + HZ- Answer Bank acid base For the given reactions, classify each reactant as a Lewis acid-base, a Brønsted-Lowry acid-base, or both.... For the given reactions, classify each reactant as a Lewis acid-base, a Brønsted-Lowry acid-base, or both.

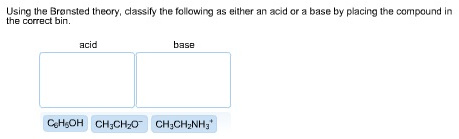
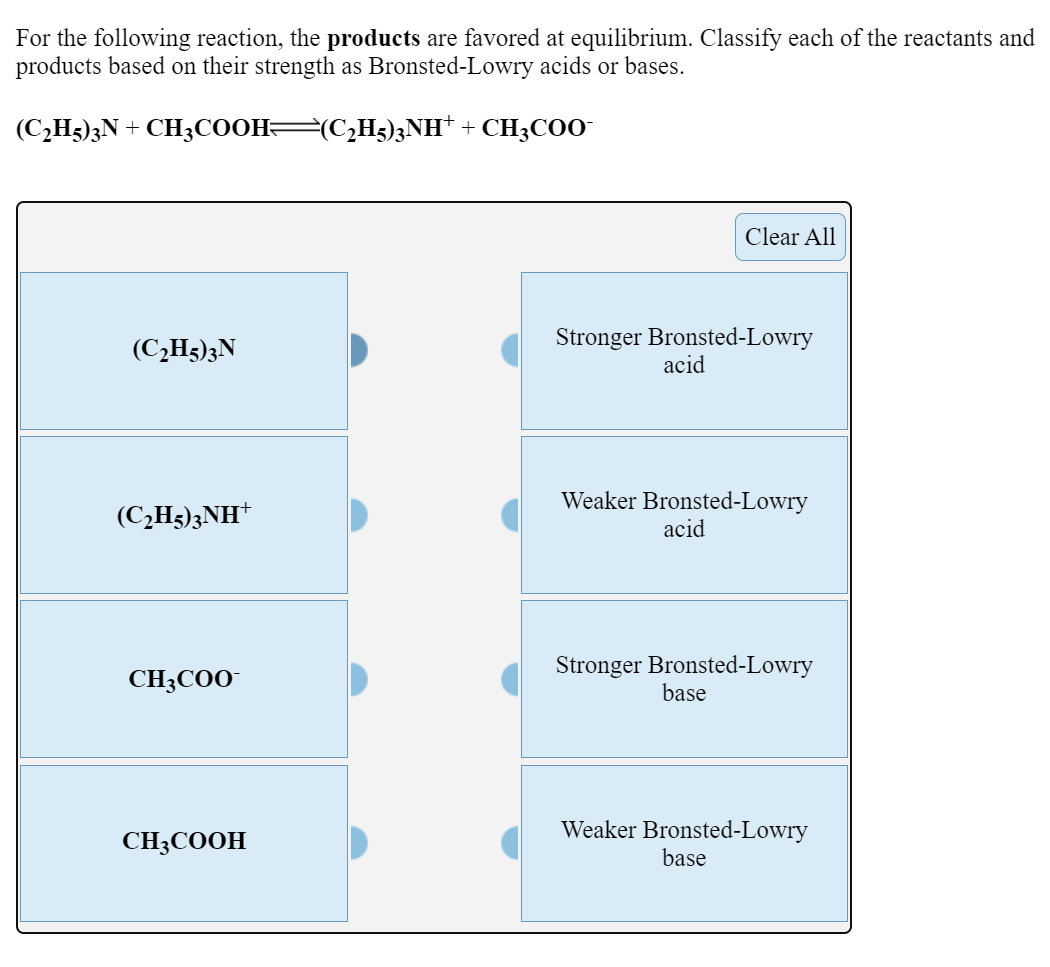
For the following, reaction, K < 1. Classify each, of the reactants and products based on their strength as, Bronsted-Lowry acids or bases., (CH3)2NH2+ + CN- , –>, (CH3)2NH + HCN, CN-, HCN, ...
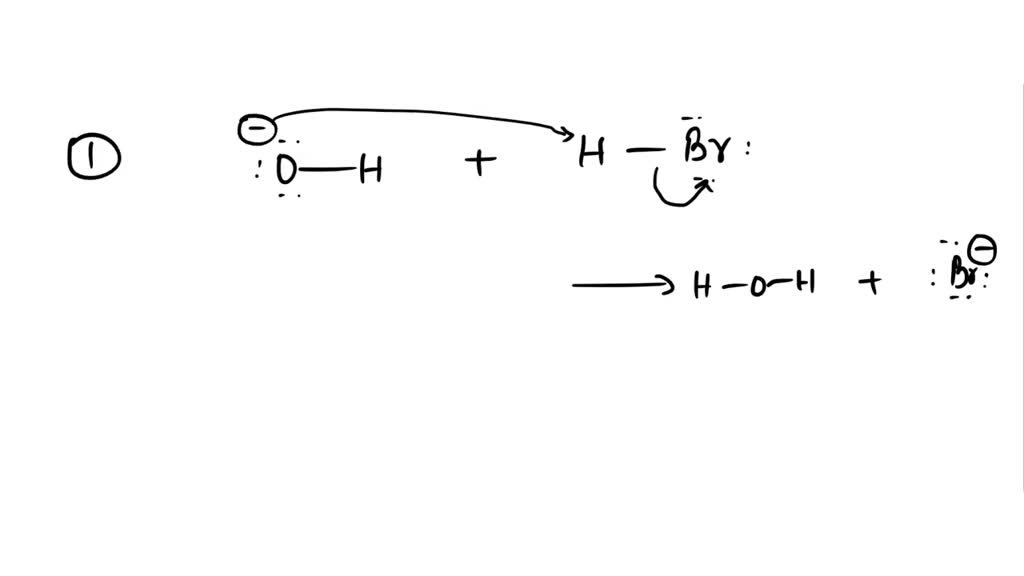
Label each reactant and product this reaction, CH,OH, HO, 1L 0'HJ, Bronsted acid base ., H,0, Assignment 2 6

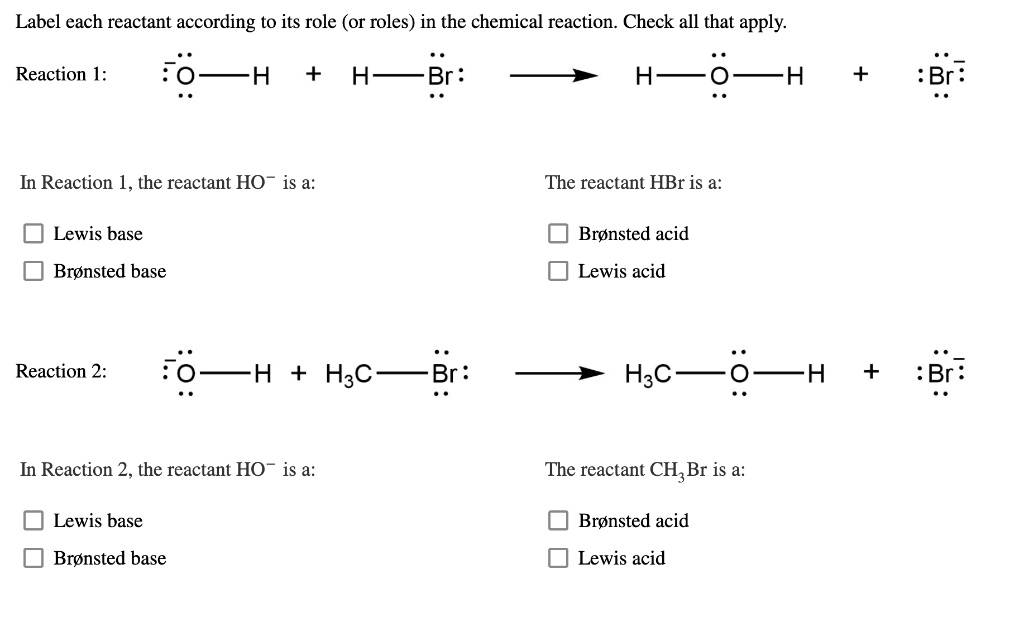



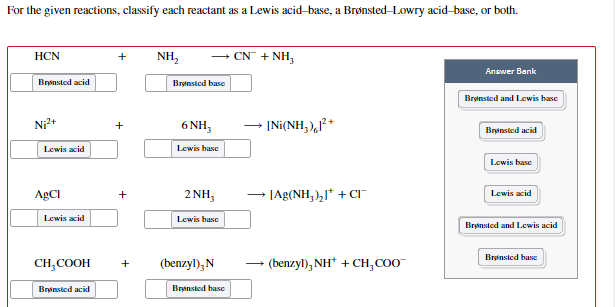



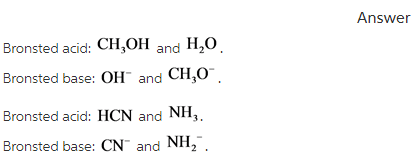


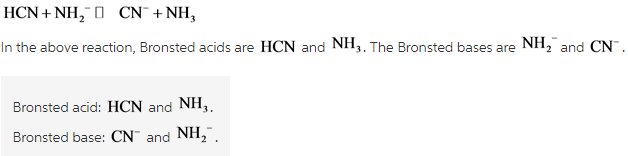
![SOLVED: 7 pis Qureuzt] reaction scheme below; Iabel each ...](https://cdn.numerade.com/ask_images/cef57db98f7b4d1bbf408a868edc6327.jpg)



0 Response to "39 label each reactant and product in this reaction as a bronsted acid or base"
Post a Comment