40 off-label drug use in pediatrics
Off-label Drug Use in Pediatrics.docx - OFF-LABEL DRUG 1... Many physicians prescribe off-label medications for certain medical conditions, symptom management and infections. Circumstances Which Children Should be Prescribed Drugs for Off-label Use In pediatrics, children should only be prescribed drugs for off-label use when the risks of not prescribing outweigh the benefits. Off-Label Prescribing in Children Remains High: A Call for Prioritized ... off-label use, prescribing behavior The US Food and Drug Administration requires that medications be tested for safety and efficacy at a specific dosage, and for a specific time period, before approval for clinical use in a particular population. 1 Use of medications outside these parameters is considered "off-label" drug use.
Off-Label Use of Drugs in Children - American Academy … Mar 01, 2014 · The term “off-label” use refers to use of a drug that is not included in the package insert (approved labeling) for that drug. The purpose of off-label use is to benefit an individual …

Off-label drug use in pediatrics
Off-Label Drug Use in Pediatric Out-Patient Care: A Multi-Center ... Results: A total of 865 drugs (mean 1 and SD 0.24) were prescribed to 326 children. Off-label was identified in 39.4% of the drugs with a frequency of 512 (as 1 drug may belong to more than 1 off-label category). The most common reason for off-label prescribing was related to doses that were "higher or lower than the recommended use" (48.6% ... Off-Label Drug Use in Pediatrics - CriticalCareNow About half the drugs used in the pediatric intensive care unit are off-label. Up to 100% of children, in some studies, receive at least one off-label drug during their hospitalization. (1,2,3) Examples of these drugs include dexmedetomidine, ketamine, and hydromorphone. Off-Label Drug Use In Pediatrics | Nursing Academia Off-Label Drug Use In Pediatrics. The unapproved use of approved drugs, also called off-label use, with children is quite common. This is because pediatric dosage guidelines are typically unavailable since very few drugs have been specifically researched and tested with children.
Off-label drug use in pediatrics. Off-label Medication Prescribing Patterns in Pediatrics: An Update Off-label drug use is defined as use in a patient younger than the lower limit of the FDA-approved age range for any indication or dosage form of that drug. RESULTS: At least 1 drug was prescribed off label in 779 270 of 2 773 770 (28.1%) patient visits during the study period. Off-label Medication Prescribing Patterns in Pediatrics: An Update Off-label drug use is defined as use in a patient younger than the lower limit of the FDA-approved age range for any indication or dosage form of that drug. Results: At least 1 drug was prescribed off label in 779 270 of 2 773 770 (28.1%) patient visits during the study period. Off-Label Use of Drugs in Children - American Academy of Pediatrics However, off-label drug use remains an important public health issue for infants, children, and adolescents, because an overwhelming number of drugs still have no information in the labeling for use in pediatrics. The purpose of off-label use is to benefit the individual patient. Off-Label Prescribing in Pediatrics - Verywell Health Off-Label Prescribing in Pediatrics. The Food and Drug Administration approved the use of the antidepressant Prozac (fluoxetine) as a treatment for children and adolescents 7 to 17 years of age with depression (major depressive disorder) or obsessive-compulsive disorder (OCD) in 2003. That new indication followed studies that showed that Prozac ...
Off-Label Drug Use in Pediatrics.docx - Running head:... Some of the off-label drugs that require extra care and attention when being used in pediatrics are antidepressants, and chronic disease drugs for instance cancer drugs. Explain strategies … Off-label use of dupilumab for pediatric patients with atopic ... Availability of the drug in 2 d … Off-label use of dupilumab for pediatric patients with atopic dermatitis: A multicenter retrospective review J Am Acad Dermatol. 2020 Feb;82(2):407-411. doi: 10.1016/j.jaad.2019.10.010. Epub 2019 Oct 10. Authors Sean Igelman 1 ... Unlicensed and Off-Label Drug Use in Children Before and After ... Off-label drug use rate was found to be between 18% and 66% for inpatients and between 10.5% and 37.5% for outpatients. After the EU Pediatric Regulation came into force in 2007, unlicensed drug use rate was found to be at 11.4% for inpatients and between 1.26% and 6.7% for outpatients. Pediatric Off-Label and Unlicensed Drug Use and Its Implications this review aims to give an overview of the worldwide reported rates of off-label and unlicensed drug use in different patient populations in pediatric settings, with a brief summary of the related adverse drug reactions (adrs) and a discussion of the existing regulatory provisions and possible solutions for ensuring safe use of medicines in …
Off-Label or Off-Limits: Should You Use a Drug for An Unapproved Use? A review published in Pediatrics states that "off-label" use of drugs, defined as when the drug is used in children but has only been FDA-approved for use in adults, is all too common. One research article estimated 45% of all of pediatric visits with prescribed drugs included off-label orders Off-Label Drug Use in Pediatrics.docx - Running head:... OFF-LABEL DRUG USE IN PEDIATRICS 3 strategies hence used to promote safety for children from infancy to adolescence is calculating the dosage from the prescribed dosage of an adult (Dooms, & Killick, 2017). The dosage is calculated in terms of age to determine the right dosage that befits every age and addresses the issue at hand. Some of the off-label drugs that require extra care and ... Recommendations on Off-Label Drug Use in Pediatric … Jun 09, 2022 · Objective: To systematically analyze the supporting evidence, drug information, and the type of off-label drug use in recommendations on off-label drug use in pediatric … Off-label use of drugs in pediatrics: a scoping review Jul 13, 2022 · To explore the current state of research on off-label drug use in children and identify the existing research gaps in this topic. Six literature databases were searched to …
Assignment: Off-Label Drug Use in Pediatrics - I am Howework Free The unapproved use of approved drugs, also called off-label use, with children is quite common. This is because pediatric dosage guidelines are typically unavailable, since very few drugs have been specifically researched and tested with children. When treating children, prescribers often adjust dosages approved for adults to accommodate a childs weight.
Off-Label Drug Use in Pediatrics - Assessment Brief Task Help Off-Label Drug Use in Pediatrics Pediatrics prescribe medication for off-label drug use for a lack of specific medication to treat a certain ailment. Healthcare providers also prescribe drugs for off-label use due to a lack of adequate clinical trials on children (Gore et al., 2017). Lack of clinical trials presents a challenge of a lack […]
Off-Label Drug Use in Pediatrics – CriticalCareNow Oct 21, 2020 · Off-label drug therapy refers to the use of a drug that varies from FDA approval in age, indication, and/or formulation. About half the drugs used in the pediatric intensive care …
Off-Label Drug Use in Pediatrics - College Pal Order Instructions Assignment: Off-Label Drug Use in Pediatrics The unapproved use of approved drugs, also called off-label use, with children is quite common. This is because pediatric dosage guidelines are typically unavailable, since very few drugs have been specifically researched and tested with children. When treating children, prescribers often adjust dosages approved for adults to […]
Off-Label Use of Generic Drugs in Pediatric Populations Off-label drug use in children generally accepted as standard medical practice, but some evidence of increased risk of adverse drug events (ADEs) 3 Generic drugs may be used off-label for ...
Off-Label Medication use in Children, More Common than … In response to this practice gap, The American Academy of Pediatrics adopted a policy statement on the use of off-label medications in children; they defined off-label use as “use of a drug that …
Off-label drugs use in pediatric palliative care - PMC Off-label use of drugs in pediatrics. On January 26th 2007, the European Regulation on medicinal products for paediatric use (Regulation EC No 1901/2006) [] entered into force in all the European Union countries.The aims of this regulation are to facilitate the development and the accessibility to medications specifically designed for children from 0 to 18 years old, to ensure that medicinal ...
Off-Label Drug Use in Pediatrics - Essayrly: Writeden.com Paper Writing ... Order Instructions Assignment: Off-Label Drug Use in Pediatrics The unapproved use of approved drugs, also called off-label use, with children is quite common. This is because pediatric dosage guidelines are typically unavailable, since very few drugs have been specifically researched and tested with children. When treating children, prescribers often adjust dosages approved for adults to
Off-Label Drug Use In Pediatrics | Nursing Academia Off-Label Drug Use In Pediatrics The unapproved use of approved drugs, also called off-label use, with children is quite common. This is because pediatric dosage guidelines are typically …
Off-label Medication Prescribing Patterns in Pediatrics: An … Off-label usage of certain medications differed between care settings. Rates of off-label medication use were higher in observational (45.5%), inpatient (53.9%), and ambulatory …
Off-Label Drug Use in Pediatrics - App Essay Writers Off-Label Drug Use in Pediatrics Pediatrics prescribe medication for off-label drug use for a lack of specific medication to treat a certain ailment. Healthcare providers also prescribe drugs for off-label use due to a lack of adequate clinical trials on children (Gore et al., 2017). Lack of clinical trials presents a challenge of a lack of ...
Off-Label Medication use in Children, More Common than We Think: A ... There is, however, a significant association between age (in years) and frequency of being prescribed an off-label drug (p<0.001). The probability of receiving an off-label prescription is reduced by 3% for every year increase in age, with the probability at less than a year old being 51.6% and the probability at 20 years old being 29.3%. Table 4:
Off-label use of dupilumab for pediatric patients with … Background: Atopic dermatitis (AD) is a common, chronic type 2 inflammatory skin disease, typically starting in infancy, with increased risk for subsequent extracutaneous atopic …
Off-Label drug use in Pediatrics | StudyGroom Off-Label drug use in Pediatrics The use of off-label drugs among children has been prevalent in clinics due to the unavailability of the appropriate dosage guidelines for the pediatrics. In most cases, prescribers adjust the adult dosage to fit the children without the knowledge that children are not just small adults, and they have different ...
Off-label use of drugs in children - PubMed However, off-label drug use remains an important public health issue for infants, children, and adolescents, because an overwhelming number of drugs still have no information in the labeling for use in pediatrics. The purpose of off-label use is to benefit the individual patient.
Off-Label Drug Use in Pediatrics - 657 Words - StudyKraken In pediatrics, off-label use is common due to multiple reasons. The primary factor that determines whether a clinician chooses to use unapproved drugs is the availability of medications that provide instructions for dosage for pediatric patients (Bazzano, Mangione-Smith, Schonlau, Suttorp, & Brook, 2009).
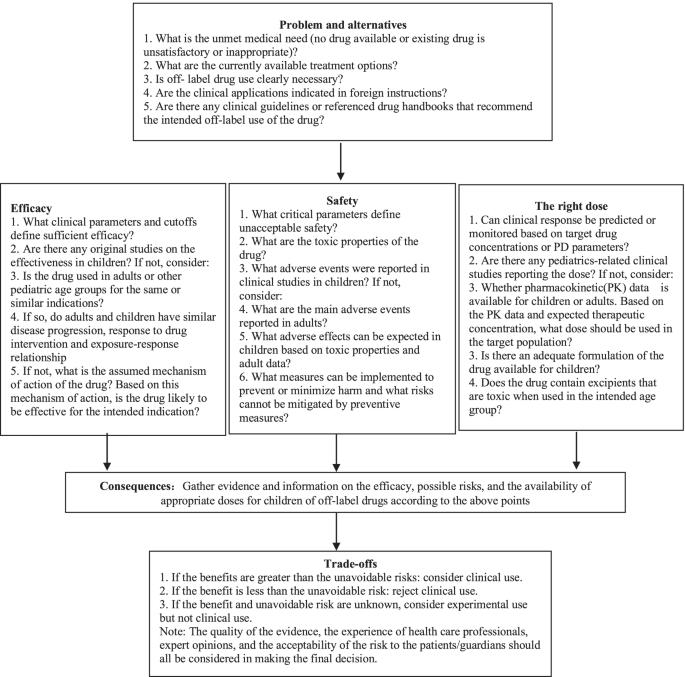
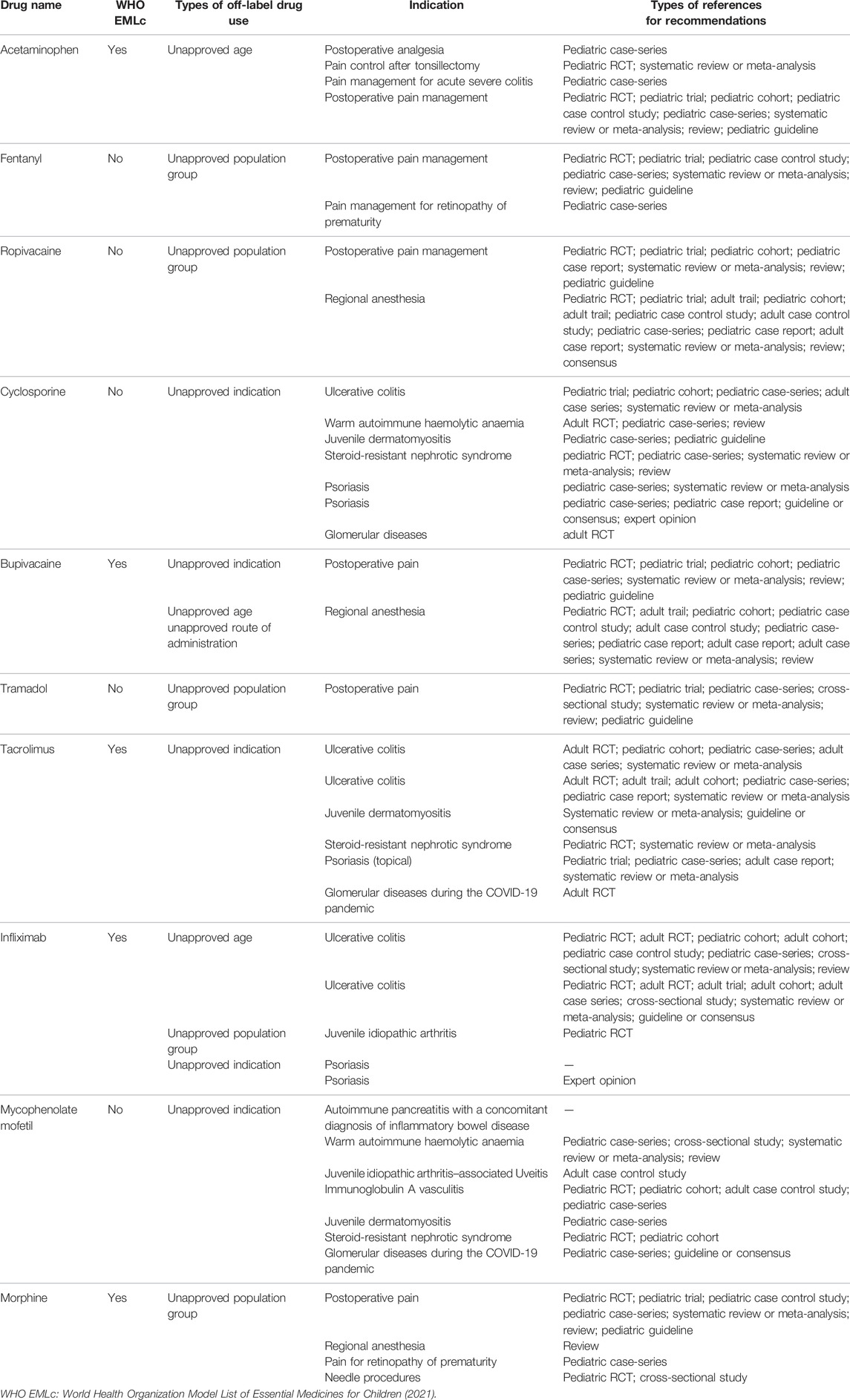
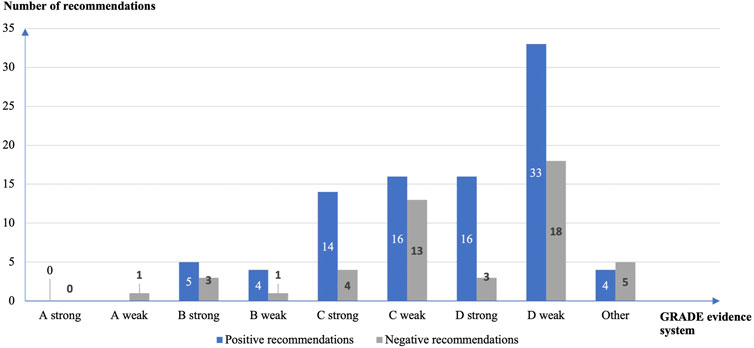
Recommendations on Off-Label Drug Use in Pediatric Guidelines Objective: To systematically analyze the supporting evidence, drug information, and the type of off-label drug use in recommendations on off-label drug use in pediatric guidelines.Methods: A cross-sectional study was performed by systematic search through MEDLINE (via PubMed) and Embase databases to identify literature published from 1 January 2018, to 31 December 2020.
Off-Label Drug Use in Pediatrics - 657 Words - StudyKraken Sep 20, 2021 · Off-label use is inescapable in pediatric care at the present moment because clinicians often have a limited choice of approved medications for children. Young patients with …
Off Label Drug Use In Pediatrics - Nursing Guys The unapproved use of approved drugs, also called off-label use, with children is quite common. This is because pediatric dosage guidelines are typically unavailable since very few drugs have been specifically researched and tested with children. When treating children, prescribers often adjust dosages approved for adults to accommodate a childs weight. However, children are not just smaller ...
Off-Label Drug Use In Pediatrics | Nursing Academia Off-Label Drug Use In Pediatrics. The unapproved use of approved drugs, also called off-label use, with children is quite common. This is because pediatric dosage guidelines are typically unavailable since very few drugs have been specifically researched and tested with children.
Off-Label Drug Use in Pediatrics - CriticalCareNow About half the drugs used in the pediatric intensive care unit are off-label. Up to 100% of children, in some studies, receive at least one off-label drug during their hospitalization. (1,2,3) Examples of these drugs include dexmedetomidine, ketamine, and hydromorphone.
Off-Label Drug Use in Pediatric Out-Patient Care: A Multi-Center ... Results: A total of 865 drugs (mean 1 and SD 0.24) were prescribed to 326 children. Off-label was identified in 39.4% of the drugs with a frequency of 512 (as 1 drug may belong to more than 1 off-label category). The most common reason for off-label prescribing was related to doses that were "higher or lower than the recommended use" (48.6% ...




















![PDF] Use of off-label and unlicensed drugs in pediatric ...](https://i1.rgstatic.net/publication/337621991_Use_of_off-label_and_unlicensed_drugs_in_pediatric_patients_A_longitudinal_prevalence_survey_from_Lahore_Pakistan/links/5de0c9af299bf10bc32edf45/largepreview.png)

![PDF] Challenges in prescribing drugs for children with cancer ...](https://d3i71xaburhd42.cloudfront.net/7194ddef9382f33a3d4bdb27431a0e413f384bd8/5-Table2-1.png)












0 Response to "40 off-label drug use in pediatrics"
Post a Comment