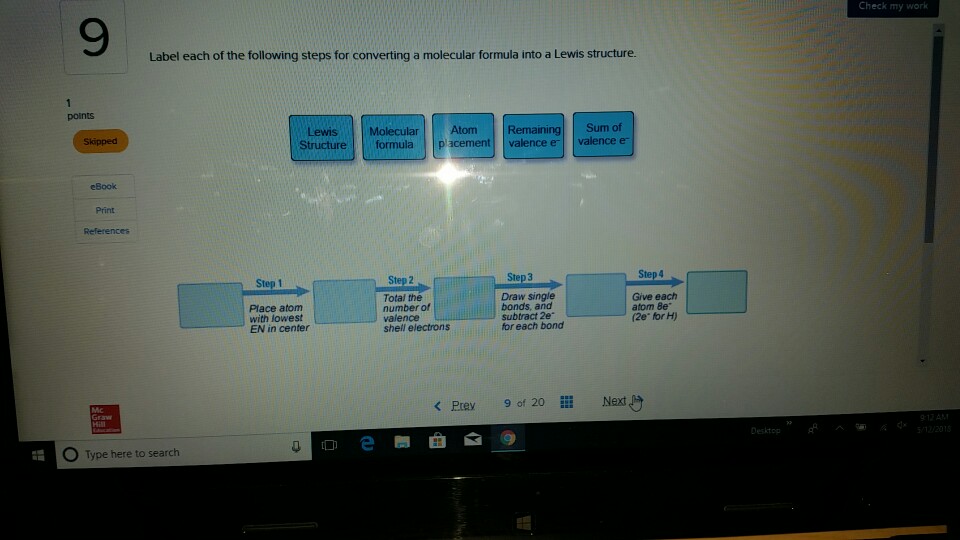
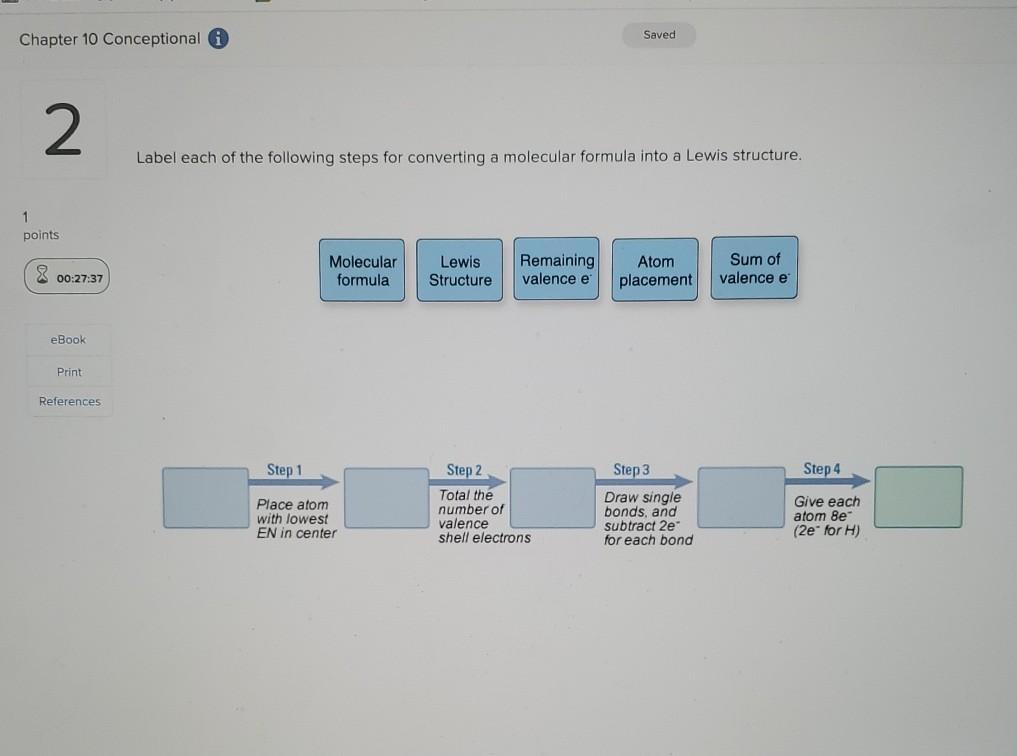
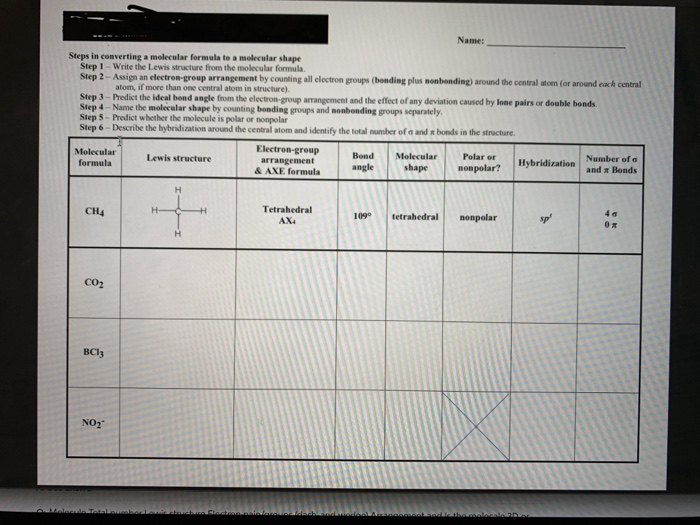
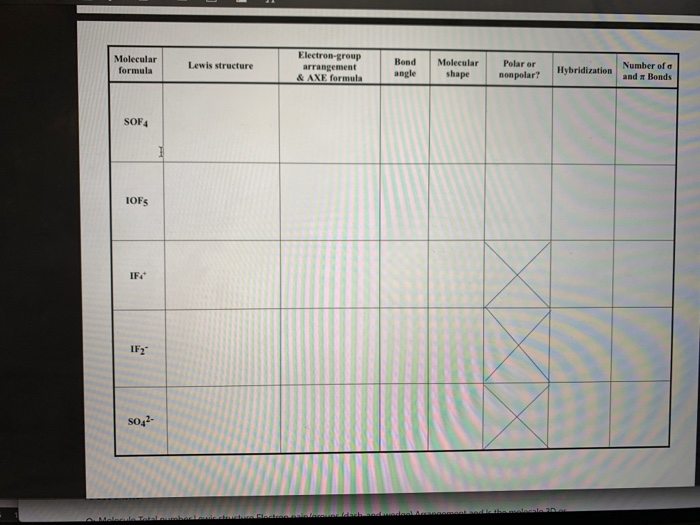
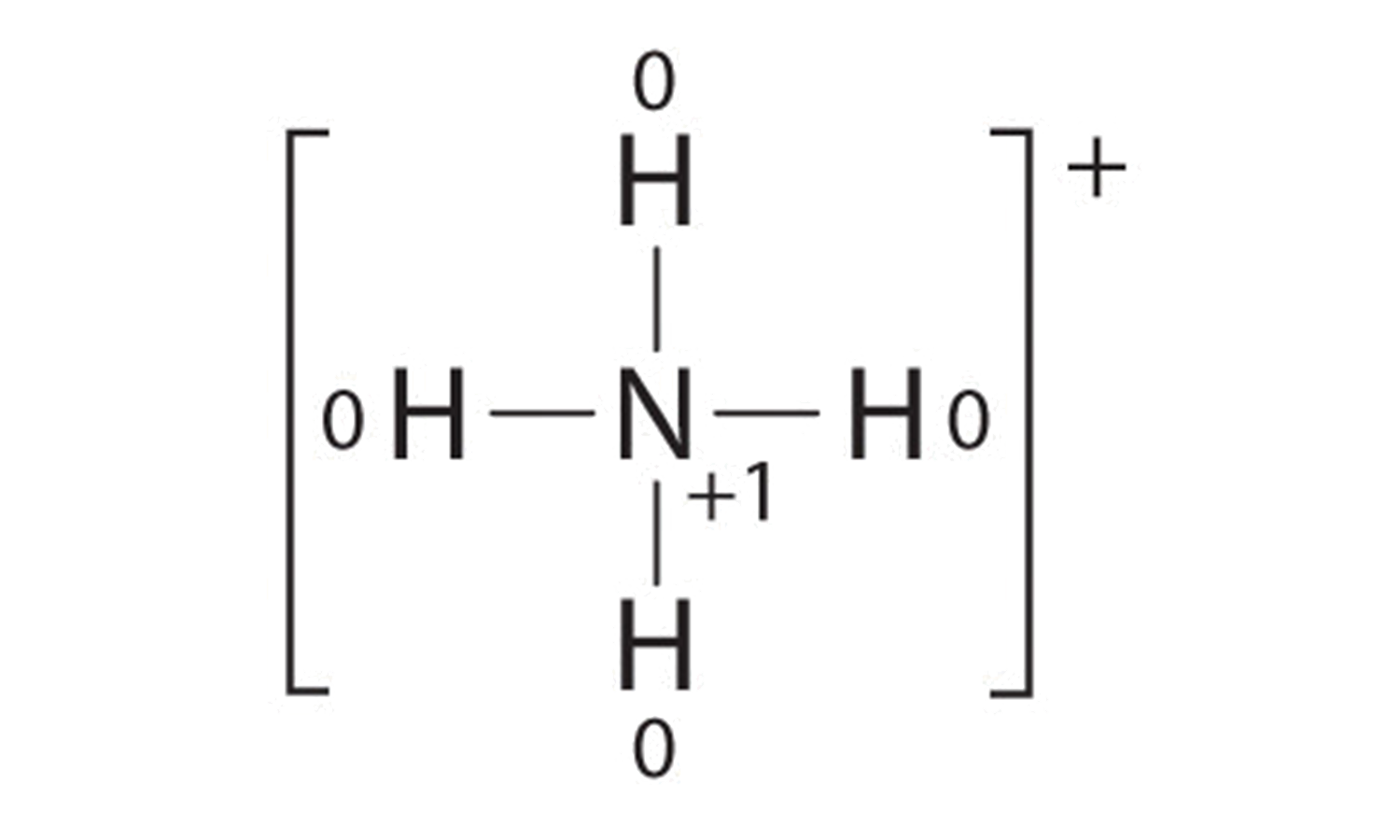
42 label each of the following steps for converting a molecular formula into a lewis structure.
Lewis Structures: Dot Symbols, How to Draw, Significance - Embibe The total number of valence electrons present in the molecule of the compound is calculated by adding the individual valence electrons of each atom. Step 2: Determine the Central Atom The least electronegative atom is chosen as the central atom of the molecule or ion. The central metal atom is the one to which all the other atoms will be bonded. Lewis Structures - Chemistry LibreTexts Lewis Structures. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron ...
Lewis Structure of H2O (Water) - Drawing Steps Lewis Structure of H 2 O (Water) - Drawing Steps. Lewis structure of water molecule contains two single bonds around oxygen atom. number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. Each step of drawing lewis structure of H 2 O are explained in this tutorial.
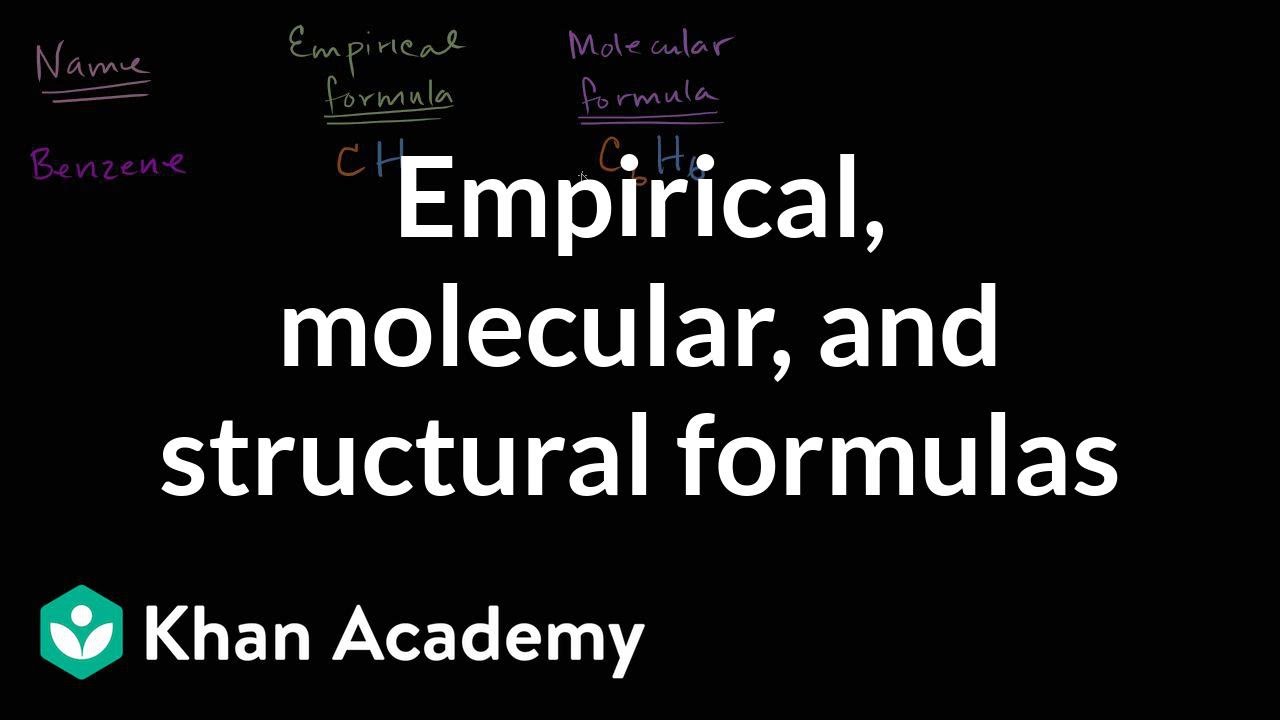
Label each of the following steps for converting a molecular formula into a lewis structure.
Q18E Question: Convert each of the fo... [FREE SOLUTION] | StudySmarter Step 1: Drawing chemical structures Skeletal structures can be drawn more easily than the condensed structures. Carbon atoms and the hydrogen atoms connected to carbon are not indicated in these structures. Atoms other than carbon and hydrogen are shown in these structures. Step 2: Converting molecular models into skeletal structures How To: Drawing Lewis Structures From Condensed Molecular Formulas There are three steps you should follow to draw a correct structure. 1. From a condensed molecular formula, you obtain information about which atoms are connected to each other in a molecule. Connect all of the appropriate atoms with single bonds first (lines). Example: CH 3 CH 2 CH 2 CO 2 CH 3 . Comment: The difficult part of this structure is deciding how to arrange the two oxygen atoms. Using the arrangement shown will produce a stable structure with filled valences for all of the atoms ... Solved Label each of the following steps for converting a | Chegg.com Draw single bonds. Subtract 2e for each bond. Place atom with lowest electronegativity in the center. Determine the total number of valence shell electrons. Give each atom 8 e (2 e for H). Step 1 Step 2 Molecular formula Step 3 Step 4 Atom placement Sum of valence e Romaining valence Lewis structure
Label each of the following steps for converting a molecular formula into a lewis structure.. Convert each condensed formula to a Lewis structure. - Numerade Here's the first siege to we need to draw three more of them in order to finish off that for on four. Okay, so ch three, followed by four siege twos and then that carbon still needs another bond. So it must be attached to this next group, which is a ch. So draw that Carmen, drop the hydrogen. Well, you still need two more bonds to this carbon. chem PROCTOR Flashcards | Quizlet 1.) place lowest EN atom in center, total valence e-, place single bonds first, distribute 8 e- to all atoms, add multiple bonds as needed. 2.) count all e- groups around central atom (A) 3.) note positions of love parts and multiple bonds. 4.) count bonding and nonbonding e- groups separately. 10.4: Writing Lewis Structures - Chemistry LibreTexts 2. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [ (2) (1) + 4 + 6] = 12 valence electrons. 3. Placing a bonding pair of electrons between each pair of bonded atoms gives the following: Six electrons are used, and 6 are left over. Solved Label each of the following steps for converting a - Chegg Label each of the following steps for converting a molecular formula into a Lewis structure. Molecular Remainin Structureformula g valence Lewis Sum of Atom valence eplacement Step 1 Step 2 Step 3 Step 4 Place atom with lowest EN in center Total the number of valence shell electrons Draw single bonds, and subtract 2e for each bond Give each atom 8e (2e for
Mastering Chemistry Set Flashcards | Quizlet One of the steps in the commercial process for converting ammonia to nitric acid is the conversion of NH3 to NO: 4NH3 (g)+5O2 (g)→4NO (g)+6H2O (g) In a certain experiment, 1.30 g of NH3NH3 reacts with 2.38 g of O2. A) Which is the limiting reactant? B) How many grams of NO and of H2O form? A) O2 B) 1.78 g, 1.60 g PHSchool.com Retirement–Prentice Hall–Savvas Learning Company About a purchase you have made. FAQs: order status, placement and cancellation & returns; Contact Customer Service Answered: Convert the following molecular model… | bartleby Solution for Convert the following molecular model into a skeletal structure. ball & stick v + labels ... Convert each shorthand structure to a complete structure with all atoms and lone pairs drawn in. a. ... Step 1: A skeletal formula is a graphical representation of the arrangement of atoms and ... Empty string - Wikipedia The empty string is a legitimate string, upon which most string operations should work. Some languages treat some or all of the following in similar ways: empty strings, null references, the integer 0, the floating point number 0, the Boolean value false, the ASCII character NUL, or other such values.
CH2Br2 Lewis Structure, Geometry, Hybridization, and Polarity Steps to Draw Lewis Structure of CH2Br2 Step 1: First of all, count the number of electrons in the valence shell of each atom. Before drawing the Lewis structure, we need to know the number of valence shell electrons on all constituent atoms and their sum. Step 2: Draw the lewis dot structure for elements. Success Essays - Assisting students with assignments online Fine-crafting custom academic essays for each individual’s success - on time. Editing. Helps students to turn their drafts into complete essays of Pro level. 26.8: From Molecular Formula to Molecular Structure If the molecular formula is given, plug in the numbers into this formula: (26.8.1) D o U = 2 C + 2 + N − X − H 2. C is the number of carbons. N is the number of nitrogens. X is the number of halogens (F, Cl, Br, I) H is the number of hydrogens. As stated before, a saturated molecule contains only single bonds and no rings. H2O Lewis Structure - Drawing Method of H2O Lewis Structure, Molecular ... Since the overall formal charge is zero, the above Lewis structure of H 2 O is most appropriate, reliable, and stable in nature.. Molecular Geometry of H 2 O. The oxygen atom forms two single sigma bonds with the hydrogen atoms in the H 2 O molecule. Although these two Hydrogen atoms are symmetrically arranged in the plane, the two lone pairs of electrons on the Oxygen atom push these atoms.
PPIC Statewide Survey: Californians and Their Government Oct 26, 2022 · Key Findings. California voters have now received their mail ballots, and the November 8 general election has entered its final stage. Amid rising prices and economic uncertainty—as well as deep partisan divisions over social and political issues—Californians are processing a great deal of information to help them choose state constitutional officers and state legislators and to make ...
Bond-line, Lewis and Condensed Structures with ... - Chemistry Steps 4) Draw the carbon chain in a zig-zag form. Putting the first atom up or down doesn't matter as long as you keep the correct connectivity of atoms. 5) Erase the carbon atoms together with the hydrogens on them. Keep all the heteroatoms (any atom except carbon) together with the hydrogens on them.
Microsoft is building an Xbox mobile gaming store to take on ... Oct 19, 2022 · Microsoft still hopes to close this deal out by spring 2023, but there’s a good chance we have months of battles ahead — as well as the opportunity to gain rare insights, as with these mobile ...
label each of the following steps for converting a molecular formula ... Get the detailed answer: label each of the following steps for converting a molecular formula into a lewis structure.
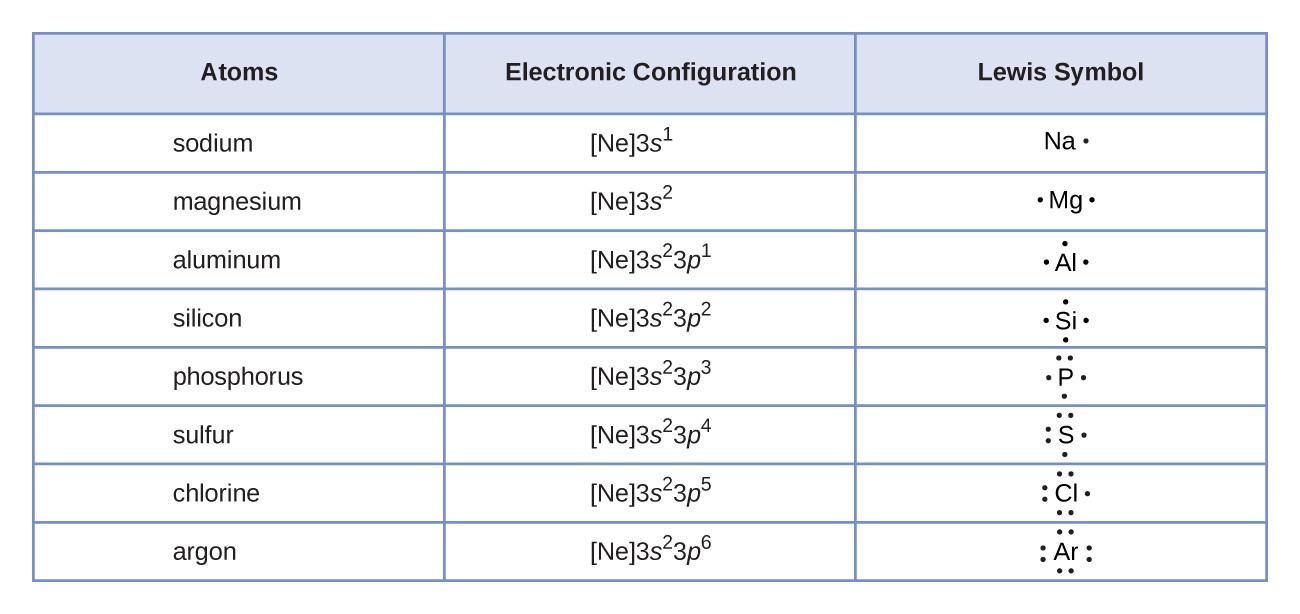
Lewis Electron Dot Structures - Detailed Explanation with ... - BYJUS Lewis dot structures reflect the electronic structures of the elements, including how the electrons are paired. Lewis structures are a useful way to summarize certain information about bonding and may be thought of as "electron bookkeeping". In Lewis dot structures each dot represents an electron. A pair of dots between chemical symbols for ...
Could Call of Duty doom the Activision Blizzard deal? - Protocol Oct 14, 2022 · On Wednesday, the U.K.’s Competition and Markets Authority, one of three pivotal regulatory bodies arguably in a position to sink the acquisition, published a 76-page report detailing its review findings and justifying its decision last month to move its investigation into a more in-depth second phase.
Chemical formula - Wikipedia An example of the difference is the empirical formula for glucose, which is CH 2 O (ratio 1:2:1), while its molecular formula is C 6 H 12 O 6 (number of atoms 6:12:6). For water, both formulae are H 2 O. A molecular formula provides more information about a molecule than its empirical formula, but is more difficult to establish.
Lewis Structures ... 100+ Lewis Structures - The Geoexchange XeO 2 F 2. Steps for Writing Lewis Structures. Find the total valence electrons for the molecule. Explain How Examples: H 2 S, NCl 3, OH -. Put the least electronegative atom in the center. Note: H always goes outside. Examples: NOCl, CF 2 Cl 2, HCN. Put two electrons between atoms to form a chemical bond. Examples: CH 4, NH 3, I 2.
Check my work 5 Label each of the following steps fo... - Biology - Kunduz check my work 5 label each of the following steps for determining a molecular shape. 3.03 points molecuar shape (ax lewis structure electron- group arrangement molecular formula bond angles ebook references step 1 draw a valid lewis structure step 2 count alle groups around the central atom (a) step 3 note positions of lone pairs and multiple …
label each of the following steps for converting a molecular formula ... label each of the following steps for converting a molecular formula into a lewis structure. Magnetochemistry label molecular nmr2
SOLVED:Label each of the following steps for converting molecular ... SOLVED:Label each of the following steps for converting molecular formula into Lewis structure. Atom placement Molecular formula Lewis Structure Step ! Step 2 Total Ihe number of valence snell electrons Step3 Draw sngle bonds and subtract Ze for each bond Stopa Place atom wiln lowest cenler Give each alom 8e (20" @or H)
N2O4 Lewis Structure, Molecular Geometry ... - Techiescientist Place the lone pair of electrons on atoms after drawing the molecular structure. Check the stability of the lewis structure and minimize the charges on the atoms. By following these steps, the lewis structure of N2O4 can be drawn as-Step 1- Determine the total number of valence electron present in each atom of the molecule. According to the ...
Lewis Structures - chemed.chem.purdue.edu The trial-and-error method for writing Lewis structures can be time consuming. For all but the simplest molecules, the following step-by-step process is faster. Step 1: Determine the total number of valence electrons. Step 2: Write the skeleton structure of the molecule. Step 3: Use two valence electrons to form each bond in the skeleton structure.
Converting chemical structure to a molecular formula There are 14 hydrogens altogether in that structure, but only 9 of them are written as such. Each corner of the pentagon represents a carbon atom and it will have 4 bonds to it. if you can only see 2 then the other 2 must have hydrogens on them. Similarly, if there are 3 bonds to carbons then there must be 1 hydrogen. 1.
Solved Label each of the following steps for converting a | Chegg.com Draw single bonds. Subtract 2e for each bond. Place atom with lowest electronegativity in the center. Determine the total number of valence shell electrons. Give each atom 8 e (2 e for H). Step 1 Step 2 Molecular formula Step 3 Step 4 Atom placement Sum of valence e Romaining valence Lewis structure
How To: Drawing Lewis Structures From Condensed Molecular Formulas There are three steps you should follow to draw a correct structure. 1. From a condensed molecular formula, you obtain information about which atoms are connected to each other in a molecule. Connect all of the appropriate atoms with single bonds first (lines). Example: CH 3 CH 2 CH 2 CO 2 CH 3 . Comment: The difficult part of this structure is deciding how to arrange the two oxygen atoms. Using the arrangement shown will produce a stable structure with filled valences for all of the atoms ...
Q18E Question: Convert each of the fo... [FREE SOLUTION] | StudySmarter Step 1: Drawing chemical structures Skeletal structures can be drawn more easily than the condensed structures. Carbon atoms and the hydrogen atoms connected to carbon are not indicated in these structures. Atoms other than carbon and hydrogen are shown in these structures. Step 2: Converting molecular models into skeletal structures










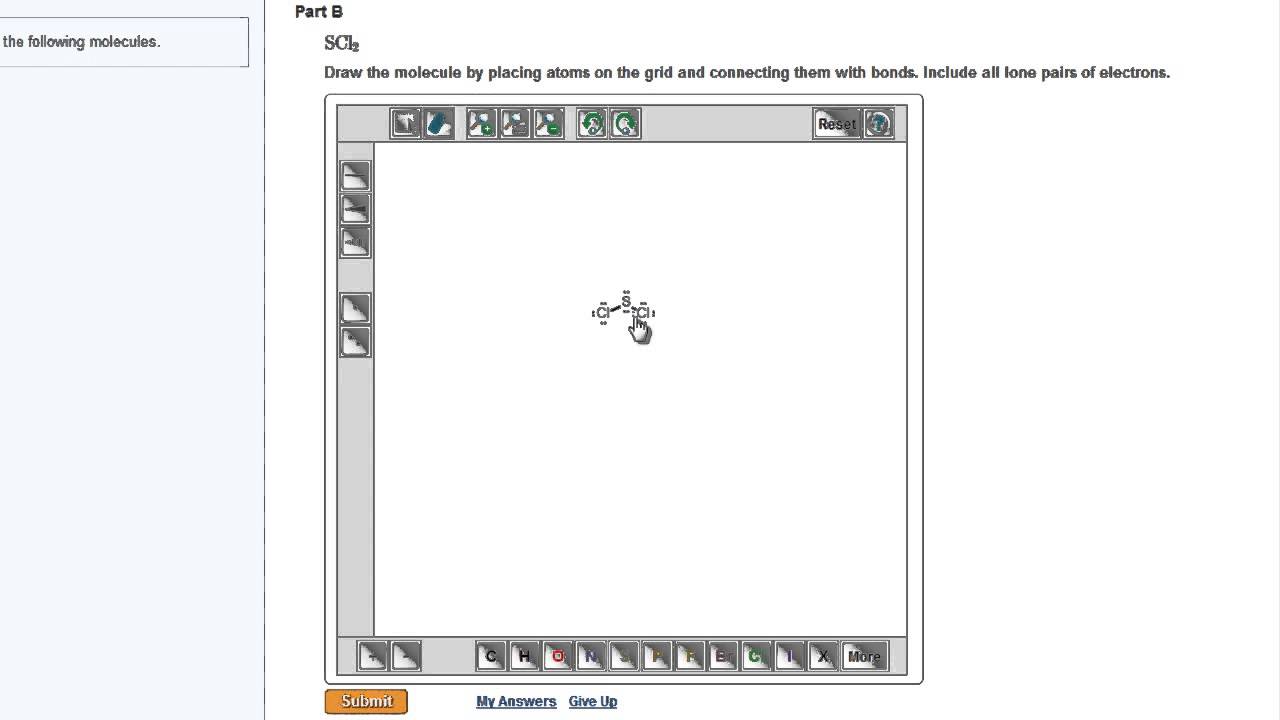

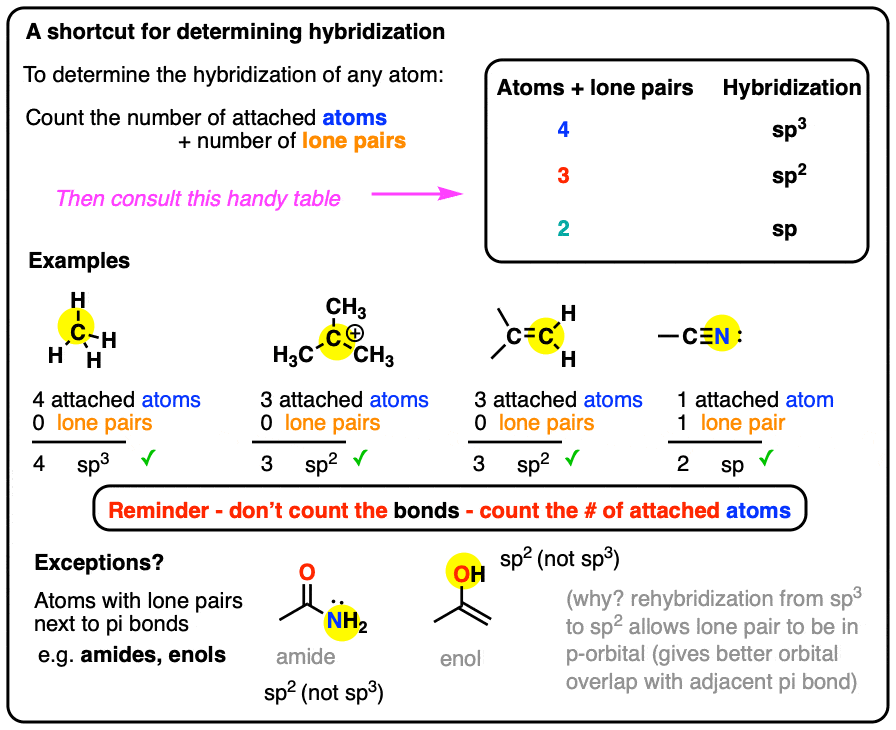
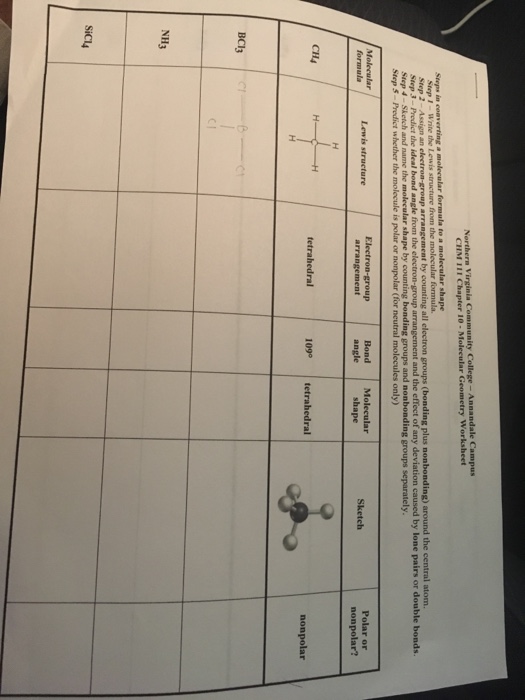












0 Response to "42 label each of the following steps for converting a molecular formula into a lewis structure."
Post a Comment