42 open label clinical trials
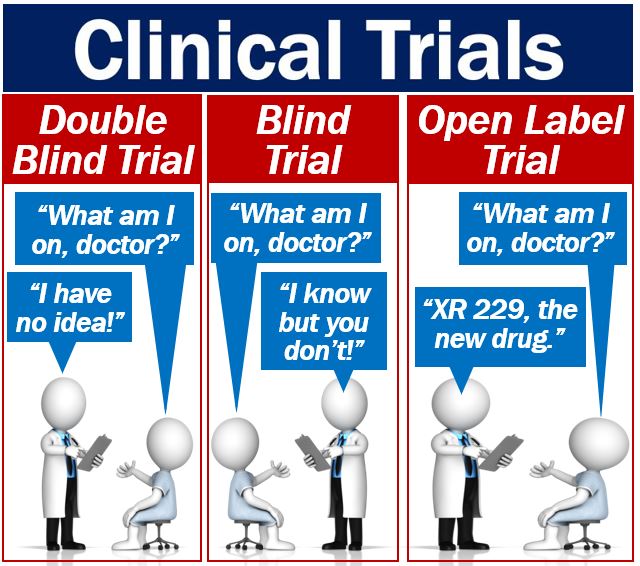
Open-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. [1] In particular, both the researchers and participants know which treatment is being administered. [1] Open-label prospective therapeutic clinical trials: oral ... - PubMed Background: Oral vancomycin (OV) in primary sclerosing cholangitis (PSC) has been evaluated as a potential therapeutic agent. We report the long-term biochemical course and outcomes of patients with PSC treated with OV. Methods: Patients were enrolled in 2 open-label clinical trials (ClinicalTrials.gov Identifier: NCT01802073 and NCT01322386) and offered OV at 50 mg/kg/day in 3 divided doses ...
Search Cancer Clinical Trials | OSUCCC - James Find a clinical trial underway at the OSUCCC - James. Search by Keyword. (i.e. John Smith, 197102, Phase 3) Filter Results. By Cancer Type. Select option. By Principal Investigator. By Study Drug. Gender.

Open label clinical trials
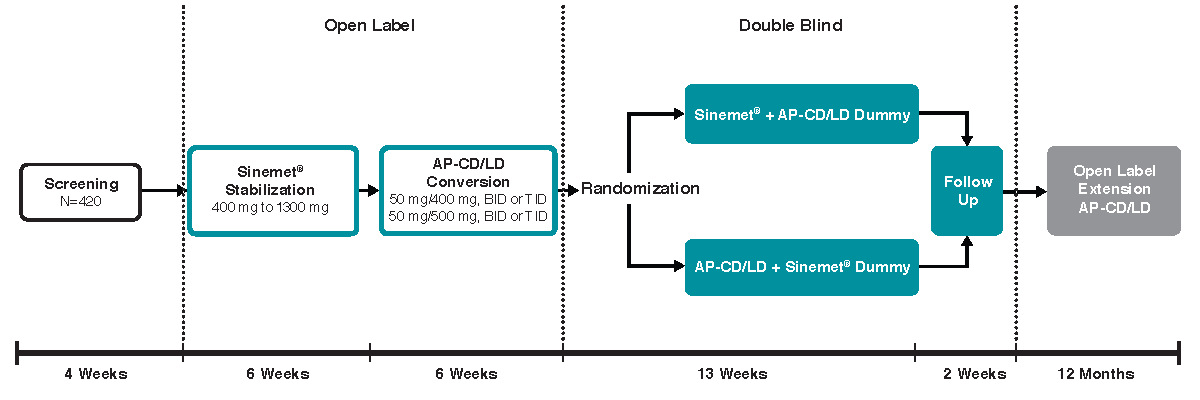
Structural Alteration of Gut Microbiota during the Amelioration of ... This study was a multicenter, randomized, positive-control, and open label clinical trial, including a 4-week washout period and a 12-week treatment period. It was performed according to the Declaration of Helsinki (2008) and approved by the Ethics Committee of Guang'anmen Hospital of the China Academy of Chinese Medical Sciences (approval no ... Open Label Expansion - Athira Clinical Trials Participating in the Open Label Extension. Upon completion of either ACT-AD or LIFT-AD study, interested participants are eligible to enroll in the Open Label Extension study of fosgonimenton. This study provides an opportunity for interested participants to receive the active drug during this study. Active participants in the OLEX Study will ... Open-label placebo clinical trials: is it the rationale, the ... However, a new programme of research in placebo studies indicates that it may be possible to harness placebo effects in clinical practice via ethical, non-deceptively prescribed 'open label placebos' ('OLPs'). To date, there have been 14 small scale clinical and experimental trials into OLPs.
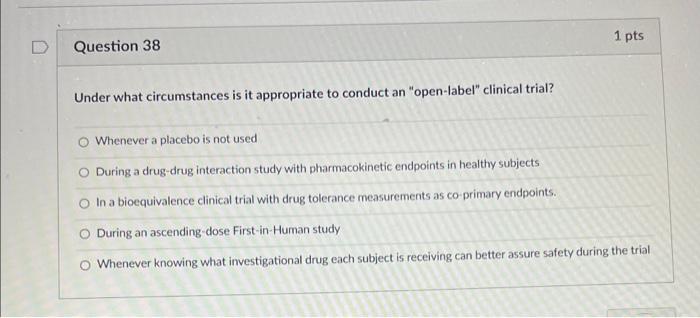
Open label clinical trials. Open-Label Clinical Trials of Oral Pulse Dexamethasone for ... - PubMed The second trial involved a more intensive regimen. Eight subjects received 4 dexamethasone doses of 50 mg/m2 every 4 weeks for 12 weeks, followed by 4 doses of 25 mg/m2 every 4 weeks for 36 weeks; subjects were randomized to 2 doses every 2 weeks or 4 doses every 4 weeks. Results: In the first trial, we enrolled 13 subjects with FSGS and 1 ... An Overview of Open Label Clinical Trials - Canada Trials An open label clinical trial is essentially one in which both the participant and the individual that is researching the drug or surgical procedure have access to information about the treatment. These trials are often used to study various medical subjects such as the effects that a new drug has on the human body, how to ensure that the drug is safe to consume, and the impact it has on a ... An Open Label Clinical Trial to Evaluate the Efficacy and ... - PubMed A 12-week open-label, single-center clinical usage trial was conducted to determine the effectiveness of a dual product regimen consisting of a 0.5% retinol treatment and an anti-aging moisturizer with 30% vitamin C in women with mild to moderate hyperpigmented and photodamaged facial skin. Clinical … PDF OPEN CLINICAL TRIALS - adventisthealth.org A Phase 1/2, Open-Label, Multi-Center, First-in-Human Study of the Safety, Tolerability, Pharmacokinetics, and Anti-Tumor Activity of TPX-0005 in Patients with Advanced Solid Tumors Harboring ALK, ROS1, or NTRK1-3 Rearrangements (TRIDENT-1 ... OPEN CLINICAL TRIALS PIPELINE.
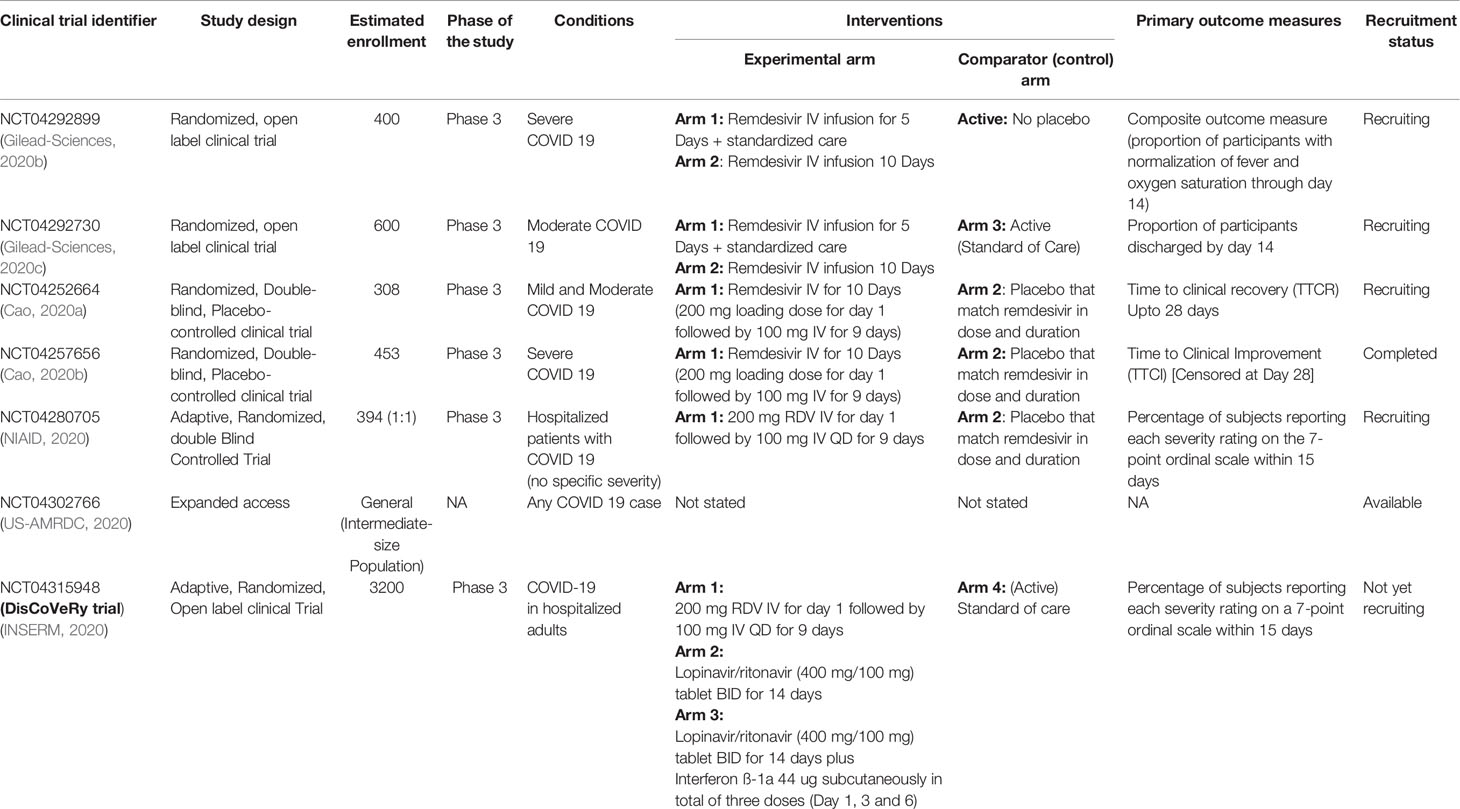
A Prospective, Open-label, Multi Cohort Clinical Study on the First ... This is a randomized, open, parallel controlled clinical study. Patients with advanced esophageal cancer who have not received any previous systemic antitumor therapy for esophageal cancer were selected to compare the efficacy and safety of carrilizumab combined with chemoradiotherapy versus carrilizumab combined with chemotherapy. Study Design Open-Label Trial | NIH - HIV.gov A type of clinical trial. In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants. Related Term(s) Clinical Trial. Double-Blind Study. Print. Print this term. Download Glossary. English Version PDF(3.13MB) Spanish Version PDF(3.16MB) CONNECT WITH US. Facebook. Multicenter, open-label, exploratory clinical trial with Rhodiola rosea ... According to its exploratory design, which aimed to evaluate the study data on a descriptive level, the trial was set up as an open-label, single-arm, multicenter study. Control groups and randomization were therefore unnecessary. The trial was conducted at four centers in Vienna (Austria): Vienna University Hospital and three GP practices. What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials can be used to gather additional safety and efficacious data on drugs on the market to increase the confidence of clinicians, patients, and clinical bodies. They can play...
Open Label Phase 2 Basket Trial With Atezolizumab and Tiragolumab in ... in this open label phase ii trial combination therapy with the anti-pd-l1 antibody atezolizumab and the anti-tigit antibody tiragolumab will be investigated in patients with localized hnscc who will undergo surgery, advanced or metastatic msi-h cancer, pd-1 resistant metastatic melanoma, and patients with a locally advanced or metastatic solid … What is an Open-Label Clinical Trial? - Regenerative Medical Group Clinical trials are a central part of the drug and medical intervention development process. Different trial designs can be used by clinical researchers, with open-label trials one such design. This article will discuss what open-label trials are. Image Credit: PopTika/Shutterstock.com. The Importance of Clinical Trials and Good Trial Design Open-label, Pivotal Clinical Trial to Confirm Efficacy and Safety of ... Open-label, Pivotal Clinical Trial to Confirm Efficacy and Safety of Autologous Grafts Containing Stem Cells Genetically Modified for Epidermis Restoration in Patients With Junctional Epidermolysis Bullosa (HOLOGENE 5) The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. A Phase 1/2 Trial of TC-510 In Patients With Advanced Mesothelin ... tc-510 is a novel cell therapy that consists of autologous genetically engineered t cells expressing two synthetic constructs: first, a single-domain antibody that recognizes human mesothelin, fused to the cd3-epsilon subunit which, upon expression, is incorporated into the endogenous t cell receptor (tcr) complex and second, a pd-1:cd28 switch …
A potential bias in safety evaluation during open-label ... - PubMed Abstract. Purpose: To describe a bias that can occur in the analysis of data from certain randomized trials. Methods and results: Although randomized trials are effective at preventing confounding, a potentially strong confounding can arise in certain therapeutic drug trials in which follow-up is extended in an open-label phase that allows ...
Understanding Clinical Trial Terminology: What is an Open Label ... Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population.
Phase 3, Open-label Clinical Trial of EB-101 for the Treatment of ... This open-label, controlled study will evaluate the efficacy and safety of EB-101 for the treatment of large, chronic, RDEB wounds. The study intervention consists of one-time surgical application of gene-corrected keratinocyte sheets (EB-101) for the treatment of RDEB wound sites in up to approximately 10-15 participants.
What is an open label trial? | The BMJ Open label trials are sometimes referred to as "non-masked" or "unblinded." If the trial is a non-pharmacological study, such as a trial of devices, or psychological and physical treatments, it may be referred to simply as "open." After recruitment to the trial, the participants were allocated to treatment using block randomisation.
Open-label placebo clinical trials: is it the rationale, the ... However, a new programme of research in placebo studies indicates that it may be possible to harness placebo effects in clinical practice via ethical, non-deceptively prescribed 'open label placebos' ('OLPs'). To date, there have been 14 small scale clinical and experimental trials into OLPs.
Open Label Expansion - Athira Clinical Trials Participating in the Open Label Extension. Upon completion of either ACT-AD or LIFT-AD study, interested participants are eligible to enroll in the Open Label Extension study of fosgonimenton. This study provides an opportunity for interested participants to receive the active drug during this study. Active participants in the OLEX Study will ...
Structural Alteration of Gut Microbiota during the Amelioration of ... This study was a multicenter, randomized, positive-control, and open label clinical trial, including a 4-week washout period and a 12-week treatment period. It was performed according to the Declaration of Helsinki (2008) and approved by the Ethics Committee of Guang'anmen Hospital of the China Academy of Chinese Medical Sciences (approval no ...
































0 Response to "42 open label clinical trials"
Post a Comment