44 fsis label approval form
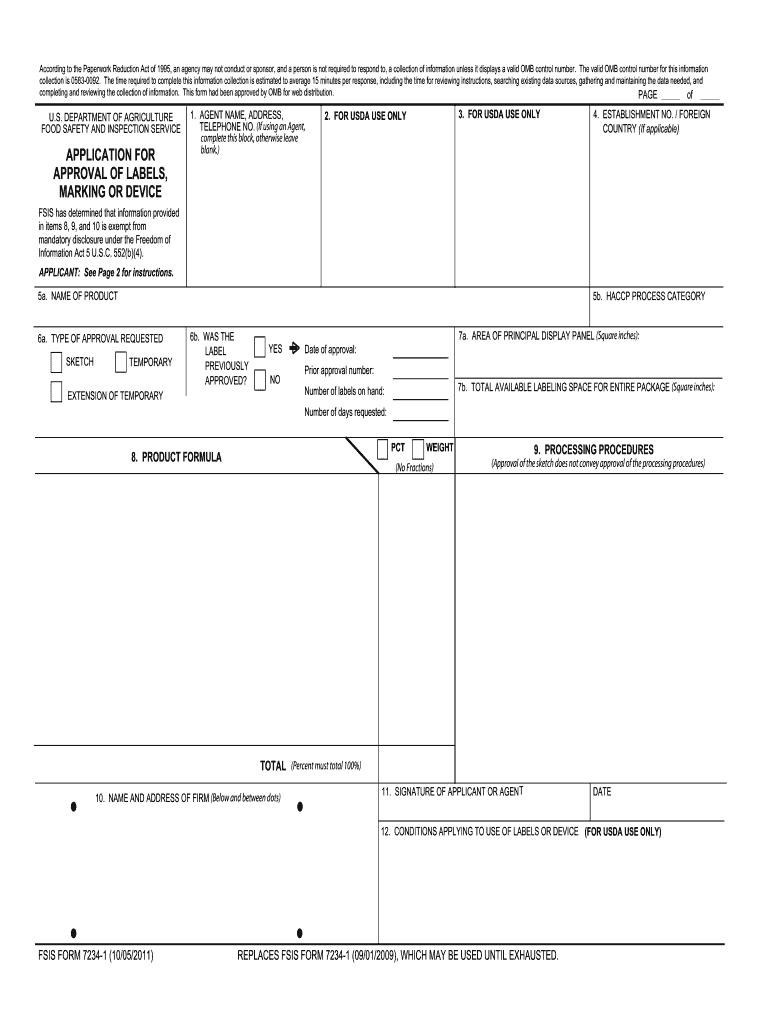
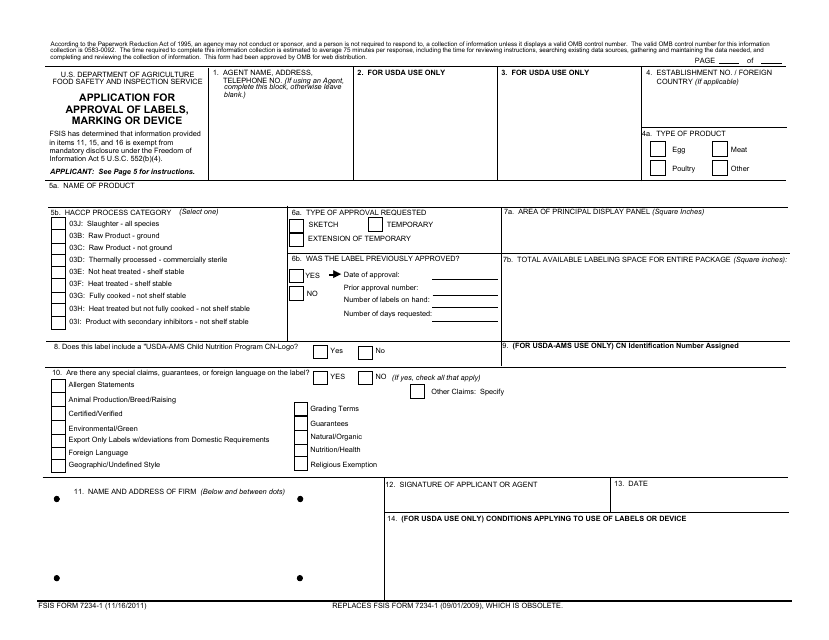
PDF FSIS 7234-1 Application for Approval of Labels, Marking or Device FSIS FORM 7234-1 (11/16/2011) REPLACES FSIS FORM 7234-1 (09/01/2009), WHICH IS OBSOLETE. 03J: Slaughter - all species 03B: Raw Product - ground. 03C: Raw Product - not ground ... This form is used to apply for approval of labels, marking or device.\r\n\r\nSubject: Public Use Form\r\nKeywords: FSIS 7234-1; Application for Approval of Labels ... PDF Step by Step Instructions for Filling Out Federal and State Special ... The plant inspection status will determine if the approval application is submitted to the state or federal labeling division. Fill out FSIS FORM 7234-1 if your processor operates under USDA or TA inspection. Before filling out the application form, check with your processor to find out if they prefer to submit the
Comprehensive List of Reasons for Label Modifications and Returns - USDA Label Application and Label Presentation: ( 9 CFR 317.4 (c)) and Instructions for Preparations for FSIS Form 7234.1 Two copies of the label must be submitted. A copy of the label must be attached to each application. To assemble the submission: Staple a copy of the label to each application. Then staple the applications together.
Fsis label approval form
Generic Label Approval | Food Safety and Inspection Service Full Guideline Generic Label Approval This guidance provides information about procedures for obtaining prior approval for sketch and generic labels for meat and poultry products. It applies to food manufacturers and official establishments seeking to request label consideration and the actions they may take before products may be marketed. Fsis Form 7234 1 ≡ Fill Out Printable PDF Forms Online The Fsis Form 7234 1 is also known as the Eligibility Certification for Export of Meat and Poultry Products form. completing this form correctly is important, as it will help ensure that your meat or poultry products are exported successfully. Make sure you have all the information you need before you start filling out this form. eCFR :: 9 CFR Part 412 -- Label Approval ( a) No final label may be used on any product unless the label has been submitted for approval to the FSIS Labeling and Program Delivery Staff, accompanied by FSIS Form 7234-1, Application for Approval of Labels, Marking, and Devices, and approved by such staff, except for generically approved labels authorized for use in § 412.2.
Fsis label approval form. Prior Label Approval System: Generic Label Approval - Federal Register (a) No final label shall be used on any product unless the label has been submitted for approval to the FSIS labeling program at headquarters, accompanied by FSIS Form 7234-1, Application for Approval of Labels, Marking, and Devices, and approved by such division, except for generically approved labels authorized for use in § 412.2. Prior Label Approval System: Generic Label Approval To obtain sketch label approval, domestic meat and poultry establishments and certified foreign establishments, or their representatives, submit sketch labels to FSIS for evaluation, except when the label is generically approved by the Agency under 9 CFR 317.5 or 381.133. FSIS Compliance Guideline for Label Approval | Food Safety and ... FSIS Compliance Guidance for Label Approval Replaces: August 2017 version This guidance document provides official establishments information about the types of labels that must be submitted to LPDS for approval. Included are specific examples of special statements and claims that must be submitted to LPDS for approval. Home | IMIC Inc | Food Label Consultants | USDA | FDA FDA Regulated Foods Order Form. Contact . ... While "generic" type labels may still go to FSIS for label approval they will be […] FDA AND IMPORTED FOODS. January 31, 2014. Under the FDA Food Safety Modernization Act (FSMA) enacted in 2011, FDA has been granted new authorities that allow the agency to better ensure the safety of food ...
Label Submission and Approval System (LSAS) | Food Safety and ... Label Submission and Approval System (LSAS) Accessing LSAS Technical Contacts LSAS Fact Sheets, Backgrounders & Articles Training Resources & Presentations Featured Resources Quarterly Enforcement Reports Review the enforcement actions FSIS has taken to ensure that consumers have access to safe, wholesome and properly labeled products. Learn More askFSIS Public Q&A: FSIS Labeling Records - USDA The labeling record must include: the final label applied to the product, product formulation, processing procedures and supporting documentation, including prior sketch approval from LPDS (if applicable). The recordkeeping requirements for labeling records are found in 9 CFR 320.1 (b) (10) and 381.175 (b) (6). 9 CFR § 412.1 - Label approval. | Electronic Code of Federal ... (a) No final label may be used on any product unless the label has been submitted for approval to the FSIS Labeling and Program Delivery Staff, accompanied by FSIS Form 7234-1, Application for Approval of Labels, Marking, and Devices, and approved by such staff, except for generically approved labels authorized for use in § 412.2. FSIS experiencing 21-day delay for label approval process FSIS is currently experiencing a delay of about 20-21 business days in evaluations for labels that require review prior to use. By providing industry with label application submission tips and suggestions via the Constituent Update, FSIS is hopeful that it will achieve a faster, more efficient label evaluation process. TIP: The fastest way to obtain a label approval is to ensure the ...
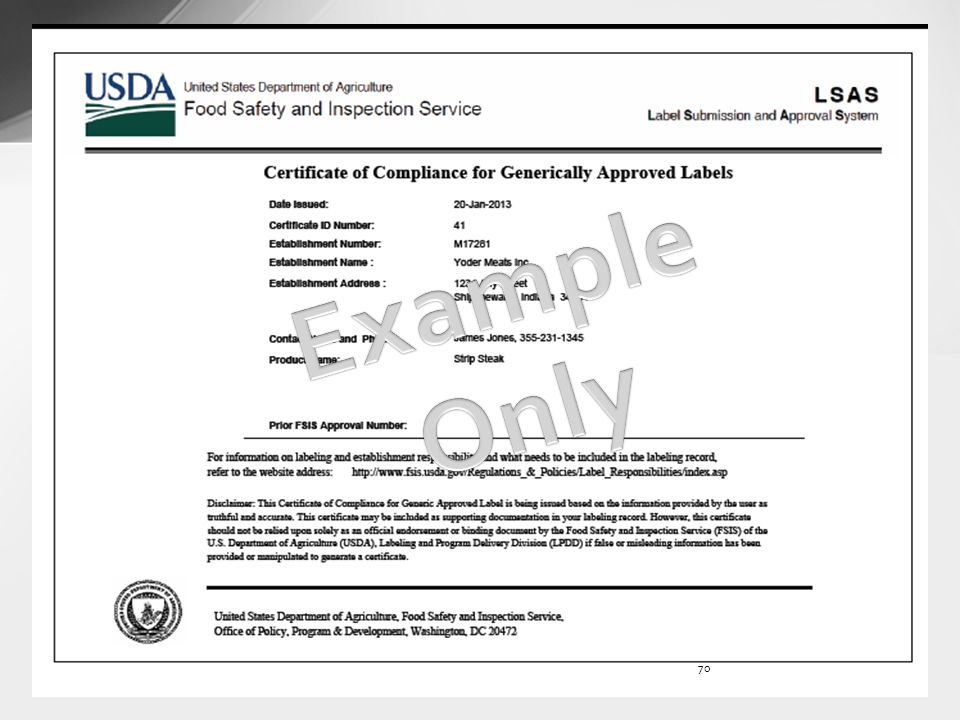
Integration of Paper Label Applications into the Label Submission and ... Type FSIS 7234-1 - Application for Approval of Labels, Marking or Device, or FSIS 8822-4 - Request for Label Reconsideration. Note: Only official forms are acceptable in the conduct of Agency business. FSIS 7234-1 is available as fill-and-print capability. Labeling and Label Approval | Food Safety and Inspection Service Labeling and Label Approval FSIS develops and provides labeling guidance, policies and inspection methods and administers programs to protect consumers from misbranded and economically adulterated meat, poultry, and egg products which ensure that all labels are truthful and not misleading. fsis form 7234 1: Fill out & sign online | DocHub Fsis label approval form. Get the up-to-date fsis label approval form 2022 now Get Form. Show details. 4 out of 5. 23 votes. The valid OMB control number for this information collection is 0583-0092. The time required to complete this information collection is estimated to average 15 minutes per response including the time for reviewing ... askFSIS Public Q&As: Generic Label Advisor Certificate - USDA Is the certificate generated by using the generic labeling advisor (GLA) in the web-based Labeling Submission and Approval System (LSAS) required for a generic label approval? No. The certificate may be included as supporting documentation in the labeling record but is not required for labels to be generically approved.
PDF FSIS Compliance Guidance for Label Approval - USDA Additional information about label approval. USDA-FSIS FSIS Compliance Guideline for Label Approval August 2017 1 ~-----_/ ( \ Preface What is the purpose of this Compliance Guideline? On November 7, 2013, the Food Safety and Inspection Service (FSIS) amended its prior
Fsis Form 7234 1 - Fill Out and Sign Printable PDF Template | signNow fsis label submission usda 7234 1 form the usda natural claims regulation does not apply to approved labels usda label portal Create this form in 5 minutes! How to create an eSignature for the fsis temporary approval Speed up your business's document workflow by creating the professional online forms and legally-binding electronic signatures.
Prime Label Consultants USDA Label Expediting I count on Prime Label for expediting our label applications through the approval process promptly. The experts at Prime Label help us identify potential issues that could delay an approval. They provide us with solid recommendations and solutions to ensure our label applications are complete, accurate and compliant.
FSIS Proposes to Address Generic Label Approvals - The National Law Review Generic label approval has been in place in some form since 1983. In a 2013 rulemaking, FSIS expanded the categories of labels eligible for generic approval and agreed to continue evaluating ...
askFSIS Public Q&A: Label Approval of Disclosure Statements ... - USDA A blanket approval refers to situations where the submission and approval of one label application covers an entire line of products without having to submit each label individually. Refer to the FSIS's compliance guide titled, "FSIS Compliance Guideline for Label Approval" for additional information on requests for blanket label approval.
Prior Label Approval System: Expansion of Generic Label Approval However, FSIS considers certain labels that comply with the Agency's labeling rules to be "generically" approved. Such labels are not submitted to FSIS, because they are deemed approved and may be applied to product in commerce. Generic label approval has been in place in some form since 1983.
Usda Label Approval: Fillable, Printable & Blank PDF Form for Free ... Click on the Get Form or Get Form Now button on the current page to make your way to the PDF editor. Give it a little time before the Usda Label Approval is loaded; Use the tools in the top toolbar to edit the file, and the change will be saved automatically; Download your edited file. Get Form. Download the form
askFSIS Public Q&A: Allergen Statement on Labels - USDA FSIS recognizes that there is a need for clear and understandable terms for use on the label that would help allergen-sensitive individuals to make Informed food choices for all foods, including meat, poultry, and egg products. Therefore, FSIS has established policies to enable processors to voluntarily add allergen statements to the labeling ...
PDF FSIS Compliance Guidance for Label Approval - Food Safety and ... FSIS Compliance Guideline for Label Approval: FSIS is publishing this compliance guideline to provide information about the types of meat and poultry product labels that need to be submitted to the Agency for approval, including specific examples of certain special statements and claims that are not generically approved.
FSIS Labeling Overview and Generic Label Approval This guidance also provides additional instruction on required labeling features, new generic labeling regulations, sample labels, label submission, and labeling records. It applies to food manufacturers and retailers and the actions they may take to comply with the label requirements applicable to meat and poultry. It relates to 9 CFR 412.
Get USDA FSIS 7234-1 2011-2022 - US Legal Forms Follow these simple instructions to get USDA FSIS 7234-1 prepared for submitting: Find the form you need in the library of legal forms. Open the document in the online editor. Read through the guidelines to learn which information you will need to include. Click the fillable fields and add the necessary information.
eCFR :: 9 CFR Part 412 -- Label Approval ( a) No final label may be used on any product unless the label has been submitted for approval to the FSIS Labeling and Program Delivery Staff, accompanied by FSIS Form 7234-1, Application for Approval of Labels, Marking, and Devices, and approved by such staff, except for generically approved labels authorized for use in § 412.2.
Fsis Form 7234 1 ≡ Fill Out Printable PDF Forms Online The Fsis Form 7234 1 is also known as the Eligibility Certification for Export of Meat and Poultry Products form. completing this form correctly is important, as it will help ensure that your meat or poultry products are exported successfully. Make sure you have all the information you need before you start filling out this form.
Generic Label Approval | Food Safety and Inspection Service Full Guideline Generic Label Approval This guidance provides information about procedures for obtaining prior approval for sketch and generic labels for meat and poultry products. It applies to food manufacturers and official establishments seeking to request label consideration and the actions they may take before products may be marketed.























0 Response to "44 fsis label approval form"
Post a Comment