44 which of the following claims could not appear on a supplement label without fda approval?
Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content... Dietary Supplement Claims: What's NOT Allowed By The FDA But there are two exceptions of drug claims that you can make, that we didn't cover in - Dietary Supplement Claims: What's Allowed - and those are: Disease claim and a Benefit claim. A disease claim can be used, if the condition of the claim is classified as a disease, and if it is the subject of an authorized health claim.
Dietary Supplements Claims, Labels and Regulations | NSF Claims In the U.S., products sold as dietary supplements are not permitted to claim that they can treat, prevent or cure a specific disease or condition. However, they can make other claims on the product label: Health Claims Disease or health claims show a link between a food or substance and a disease or health-related condition.

Which of the following claims could not appear on a supplement label without fda approval?
Which of the following claims could NOT appear on a supplement label ... All of the following statements are true of herbal supplements EXCEPT: they can be more dangerous than synthetic supplements. they may contain unknown and uncharacterized substances. the term "natural" is regulated by the FDA when used on an herbal supplement. they can have druglike effects. 17. Combined oral contraceptive pill - Wikipedia Cancer Decreased risk of ovarian, endometrial, and colorectal cancers. Usage of combined oral concetraption decreased the risk of ovarian cancer, endometrial cancer, and colorectal cancer. Two large cohort studies published in 2010 both found a significant reduction in adjusted relative risk of ovarian and endometrial cancer mortality in ever-users of OCs compared with never-users. 8 Ways to Bring a Product to Market Without FDA Approval Not all products have to meet the U.S. Federal Drug Administration's (FDA) approval: certain biologics and dietary supplements do not require it, and there are alternative pathways for OTC drugs and other products to skip the process. Bypassing FDA Approval and Bringing Your Product to Market Faster
Which of the following claims could not appear on a supplement label without fda approval?. "Major" drug labeling changes that require FDA prior approval The following quotation delineates what the FDA considers "major" changes to labeling that: (1) require pre-approval, and (2) can't be done via CBE. Any proposed change in the labeling ... Introduction to Food Product Claims — FDA Reader There are two types of health claims that appear on food labels and marketing. They are: Authorized Health Claims Qualified Health Claims Requirements for a Health Claim Health claims cannot be made about the diagnosis, cure, mitigation or treatment of diseases (this is a drug claim) They must be complete, truthful and not misleading. Why "Keto" Claims Cannot Appear On USDA-Inspected Packaging Why the Word "Keto" Cannot Be On the Packages. For the above-mentioned reasons, the word "Keto" simply cannot be added to any packaging of meat or poultry food products. Additionally, "low carb" or "zero-carb" claims are also strictly regulated by the FSIS. To give meat and poultry food brands a better idea of how the FSIS ... CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (iii) The following sample label illustrates the provisions in paragraphs (c) and (d) of this section, including paragraph (d)(10) of this section, which permits modifications for small packages: (iv) The following sample label illustrates the provisions in paragraphs (c) and (d) of this section for a drug product marketed with cosmetic claims:
Understanding Dietary Supplement Claims | Consumer Healthcare ... - CHPA In fact, the FDA has authorized only 12 health claims since 1990. To allow more health-related information on product labels, FDA established rules for the use of qualified health claims (QHC), which require less scientific evidence than authorized health claims. Unlike authorized health claims, FDA does not "approve" qualified health claims. [FACS 10] - Chapter 12 Summative Quiz Flashcards | Quizlet Correct; this statement claims to treat one of the symptoms of diabetes, and is not allowed without FDA approval. The FDA routinely monitors supplements for: a. content levels of the listed ingredients. b. the presence of contaminants. c. reports of adverse reactions. d. the truthfulness of structure/function claims. FDA Label Search Unapproved Drugs: Drugs Marketed in the United States that Do Not Have Required FDA Approval , where information about unapproved human drugs products is available. Unapproved Drugs: Drugs... Federal Preemption of 'Structure/Function' Claims on Dietary ... Federal law offers some protections to dietary supplement manufacturers against class actions claiming label statements are false and misleading. December 11, 2020 at 12:00 PM 1 minute read
FDA Allows Enhanced Claims for Vitamins and Herbal Remedies - TheRubins The FDA started to allow companies to make "qualified claims" following court rulings that the agency could not block the companies from putting true statements on labels. Health claims characterize the relationship between food, or a nutrient, and a disease or health condition. ... This was the result of the 1998 ruling by FDA that defined ... CH 12 Quiz Flashcards | Quizlet Which of the following claims could NOT appear on a supplement label without FDA approval? green tea extract Which of the following IS a botanical ingredient but is NOT an herbal ingredient? d. A cautionary statement must appear when there are known adverse effects. Which of the following is NOT true about the labeling of supplements? 5 Mistakes in Dietary Supplement Labeling that Could Land you in FDA or ... While FDA's current practice is normally to issue an FDA warning letter to a dietary supplement manufacturer or distributor who has made unlawful claims, violated GMP practices, or otherwise broken FDA law, FTC can shut down the company and force the company to disgorge illegal profits. Health Claims on Food Labels - LabelCalc Health Claims on Food Labels: LabelCalc LabelCalc Overview demo What Needs to Be on an FDA Nutrition Label? The FDA Made These Changes to The Nutrition Fact Labels in 2022 How to Tackle FDA Regulations as a New Food Business
Label Claims for Conventional Foods and Dietary Supplements A Food Labeling Guide - Appendix C: Health Claims. A "health claim" by definition has two essential components: (1) a substance (whether a food, food component, or dietary ingredient) and (2) a...
Dietary Supplements: An Advertising Guide for Industry The advertiser should not base a claim on these studies. Example 18: The marketer of an herbal supplement claims that its product promotes healthy vision and is approved in Germany for this purpose. The product has been used extensively in Europe for years and has obtained approval by the German governmental authorities, through their monograph ...
Avoid common FDA mistakes marketing health products - Cohen Healthcare Law Dietary supplements ordinarily do not need to be proven safe and effective before brought to market, provided that no disease claims are made, and the dietary supplements do not contain a new dietary ingredient (NDI) which requires FDA approval. This why if you can, market a dietary supplement, not a drug or device. Coda: Substantiate Your Claims
Skin, Hair, and Nail Supplements: Marketing and Labeling Concerns No U.S. Food and Drug Administration (FDA) approval is required to produce and market a dietary supplement. For manufacturers, there is no need to show proof of safety or efficacy prior to sale [ 4 ]. Since no approval is required, there is no centralized database of currently available dietary supplements.
Do You Need FDA Approval to Sell Supplements? - SMP Nutra The Safety of a Dietary Supplement As mentioned before, a firm doesn't need approval from the FDA before they market a product. Unlike drug products, supplements don't undergo testing to prove safe or effective before marketing or reaching the consumer. Thus, the seller has complete responsibility for the safety of its products.
Spotlight D Quiz.docx - Item: Score: Due: Submitted:... Which of the following claims could NOT appear on a supplement labelwithout FDA approval? Upload your study docs or become a Course Hero member to access this document Continue to access End of preview. Want to read all 4 pages? Upload your study docs or become a Course Hero member to access this document Continue to access Term Fall Professor
8 Ways to Bring a Product to Market Without FDA Approval Not all products have to meet the U.S. Federal Drug Administration's (FDA) approval: certain biologics and dietary supplements do not require it, and there are alternative pathways for OTC drugs and other products to skip the process. Bypassing FDA Approval and Bringing Your Product to Market Faster
Combined oral contraceptive pill - Wikipedia Cancer Decreased risk of ovarian, endometrial, and colorectal cancers. Usage of combined oral concetraption decreased the risk of ovarian cancer, endometrial cancer, and colorectal cancer. Two large cohort studies published in 2010 both found a significant reduction in adjusted relative risk of ovarian and endometrial cancer mortality in ever-users of OCs compared with never-users.
Which of the following claims could NOT appear on a supplement label ... All of the following statements are true of herbal supplements EXCEPT: they can be more dangerous than synthetic supplements. they may contain unknown and uncharacterized substances. the term "natural" is regulated by the FDA when used on an herbal supplement. they can have druglike effects. 17.






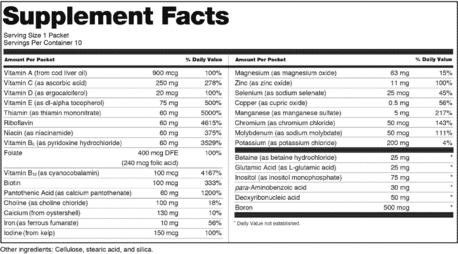


















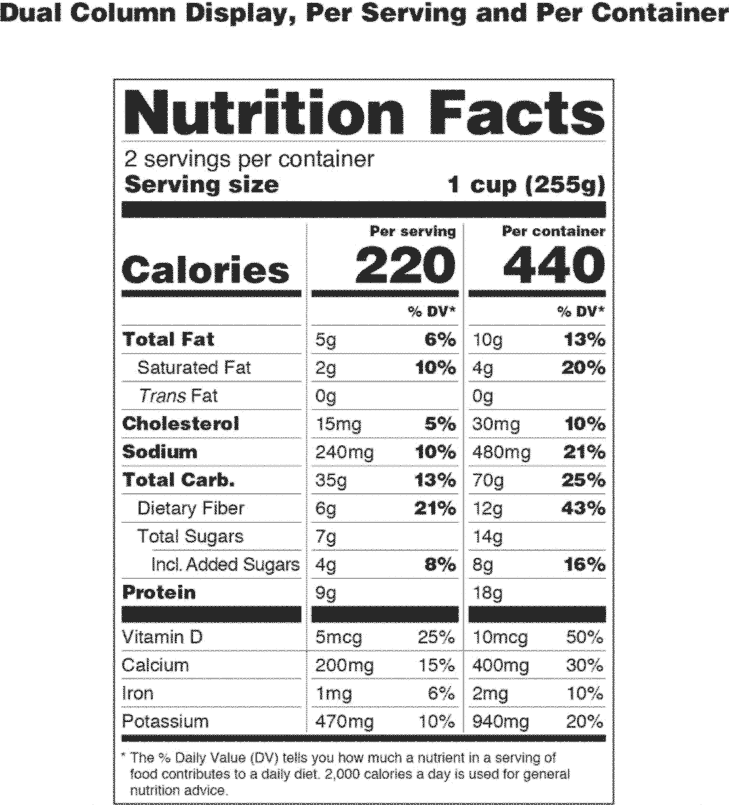





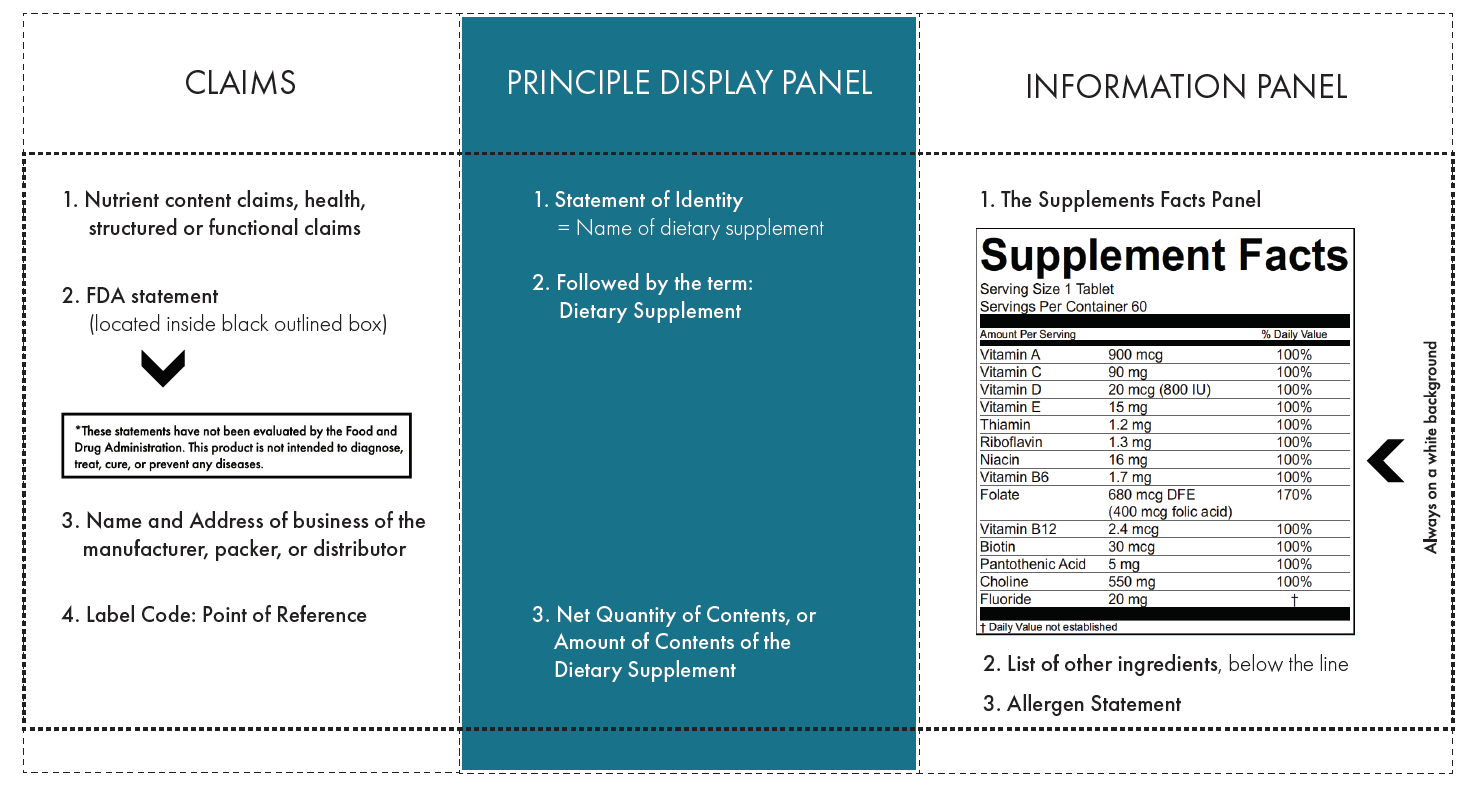



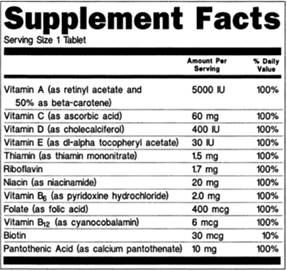
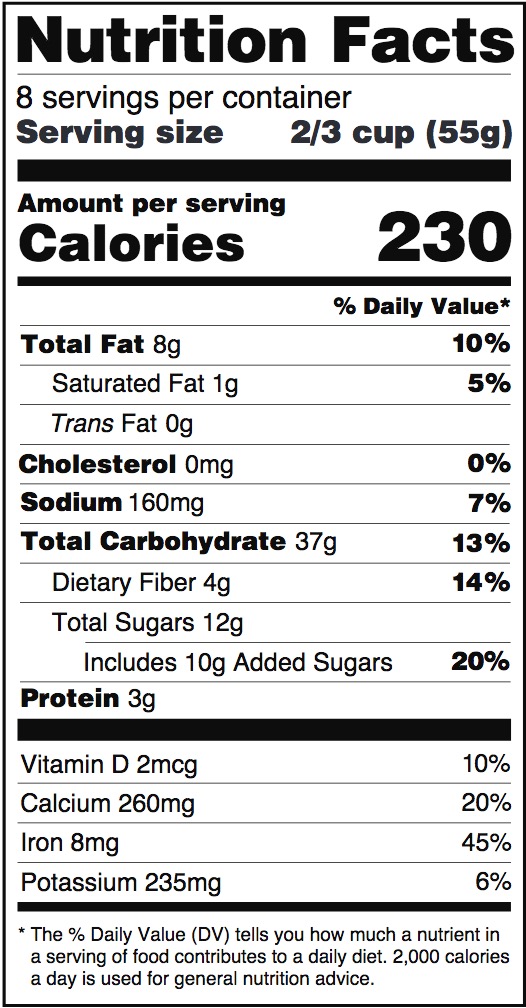
0 Response to "44 which of the following claims could not appear on a supplement label without fda approval?"
Post a Comment