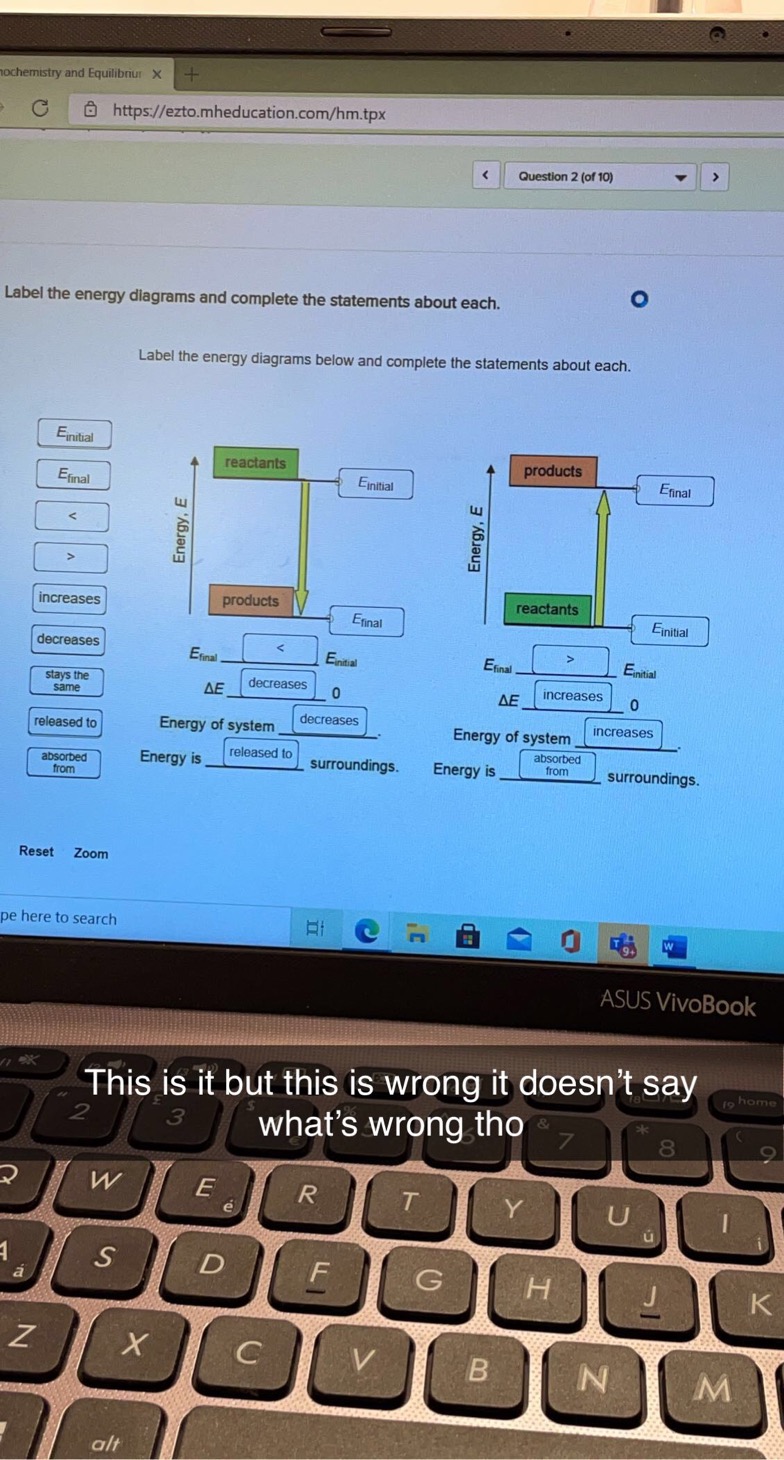
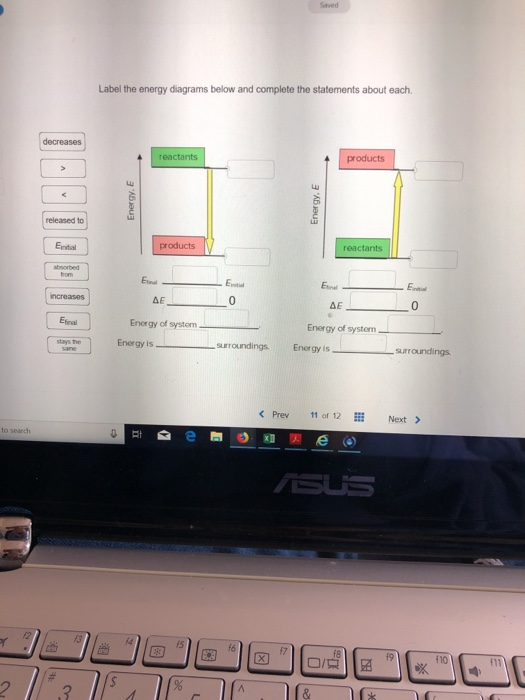
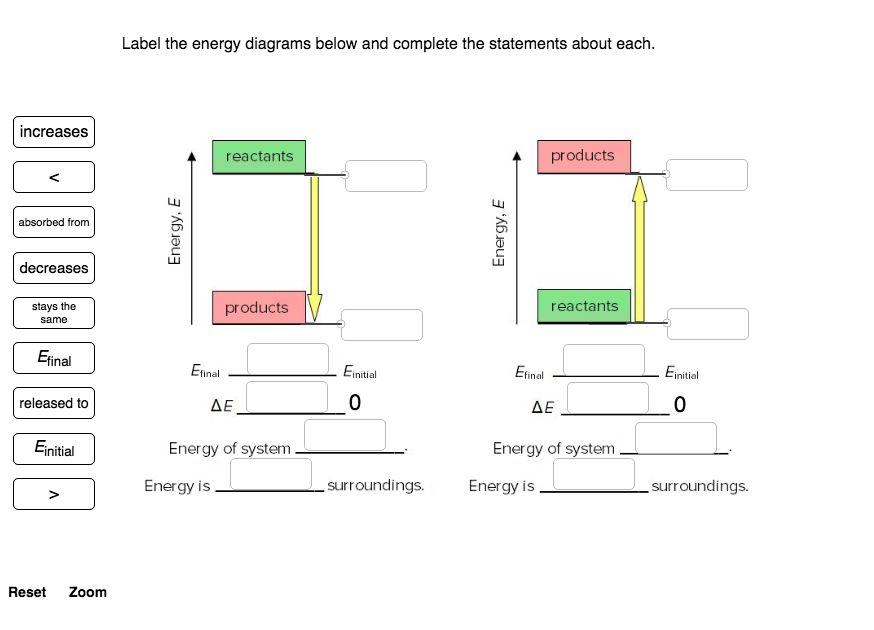
40 label the energy diagrams below and complete the statements about each
Solved Label the energy diagrams below and complete the - Chegg Transcribed image text: Label the energy diagrams below and complete the statements about each. reactants products released to stays the same Energy, E Energy, E decreases products reactants increases Efinal Einitial Efinal Einitial Einitial AE ΔΕ Jo Efinal Energy of system Energy of system absorbed from Energy is surroundings. Solved Label the energy diagrams below and complete the | Chegg.com Question: Label the energy diagrams below and complete the statems about each. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Label the energy diagrams below and complete the statems about each. Show transcribed image text Expert Answer 98% (44 ratings)
3.7: Energy Diagrams - Physics LibreTexts Elastic Force. We take precisely the same steps to draw the energy diagram for a mass on a spring, but there are some differences, such as two forbidden regions and a different slope for every position, and there is one additional feature for this potential that doesn't exist for the case of gravity: an equilibrium point.. Figure 3.7.3 - Energy Diagram for Object Influenced by Elastic Force
:max_bytes(150000):strip_icc()/phase-changes-56a12ddd3df78cf772682e07.png)
Label the energy diagrams below and complete the statements about each
Label the energy diagrams below and complete the statements about each. Label the energy diagrams below and complete the statements about each. + 20 Watch For unlimited access to Homework Help, a Homework+ subscription is required. John Edward Cayas Lv10 27 Sep 2020 Unlock all answers Get 1 free homework help answer. Already have an account? Log in Like Home Homework Help 3,800,000 Chemistry 830,000 Check 1 Label the energy diagrams below and complete the statements ... Check 1 Label the energy diagrams below and complete the statements about each. 3.57 points stays the same reactants products eBook References Finitial Energy, E Energy, E decreases increases products reactants Efinal Elina Einitial Ennal Einitial released to AE 0 AE 0 Energy of system Energy of system Energy is absorbed from surroundings. Solved Label the energy diagrams below and complete the - Chegg Question: Label the energy diagrams below and complete the statements about each. Etinal reactants products increases w Einitial Energy. E Energy, E absorbed from products reactants released to Etial Entit Etinal Einti ΔΕ 0 ΔΕ 0 decreases Energy of system Energy of system Energy is ways the Energy is surroundings surroundings same Reset Zoom
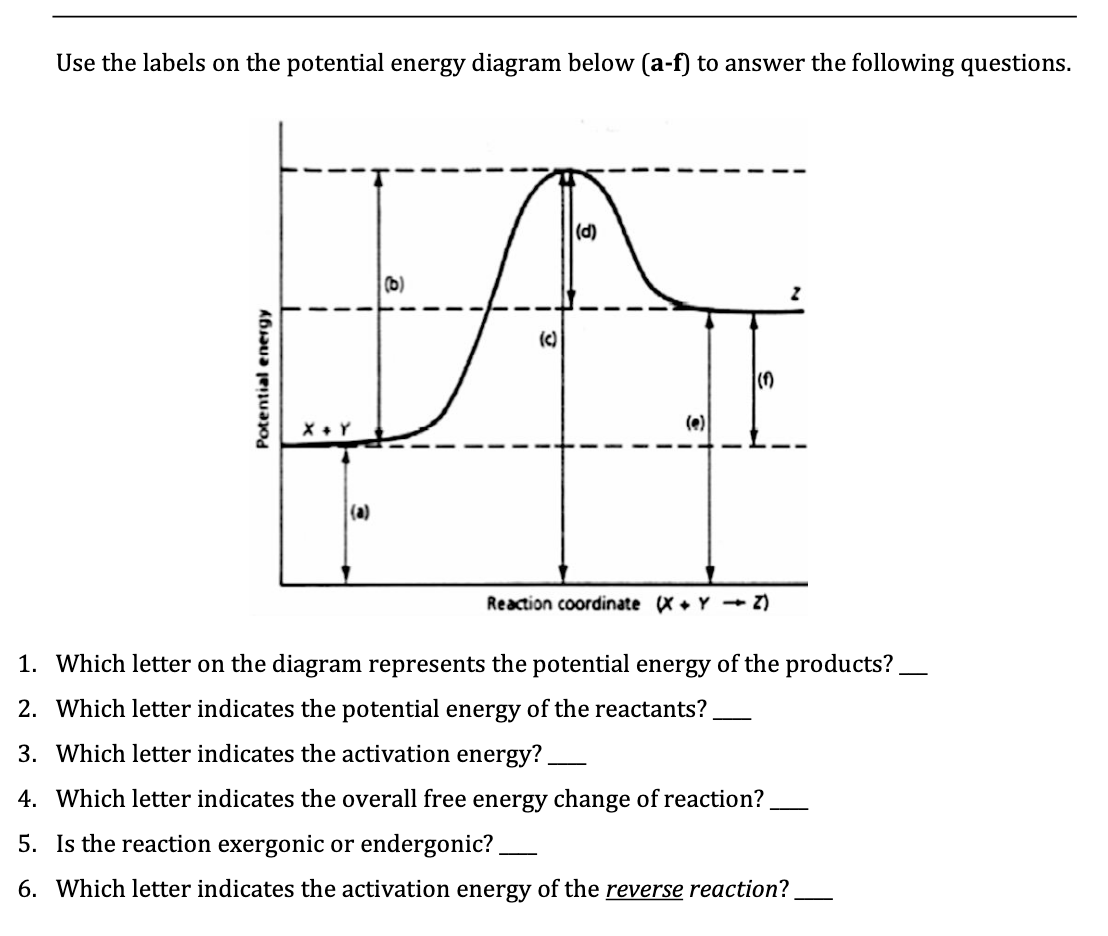
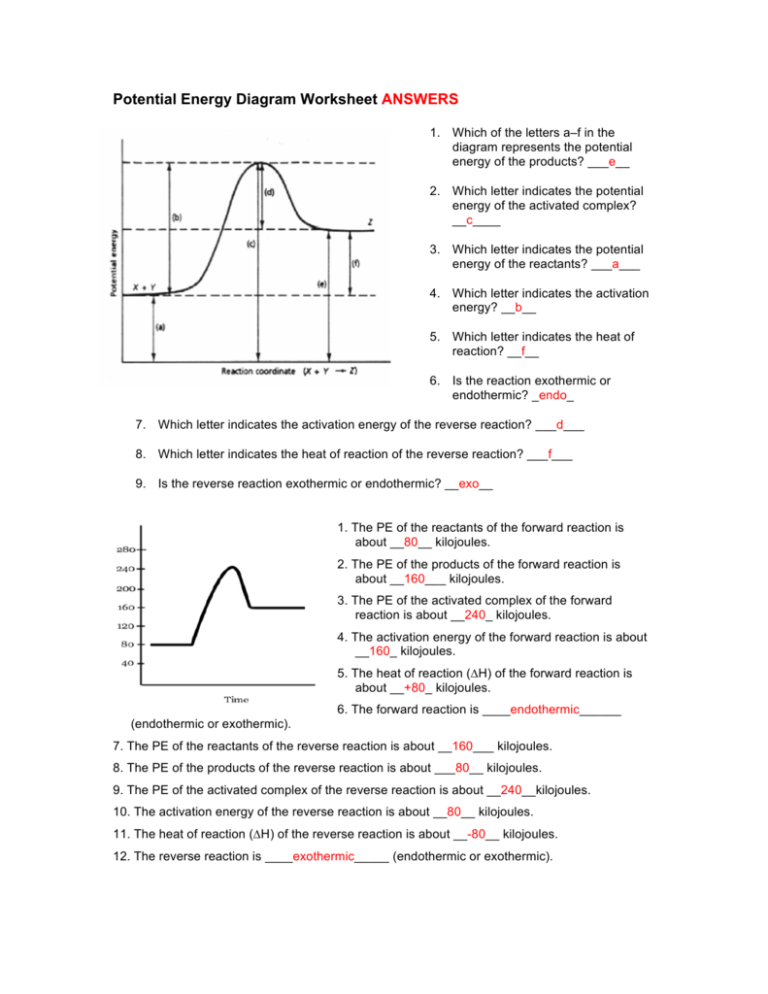
Label the energy diagrams below and complete the statements about each. Potential Energy Diagrams - Kentchemistry.com A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram. Sometimes a teacher finds it necessary to ask questions about PE diagrams that involve actual Potential Energy values. HW Solutions #9 - Chemistry LibreTexts 1. Answer the following questions based on the potential energy diagram shown here: Does the graph represent an endothermic or exothermic reaction? Label the position of the reactants, products, and activated complex. Determine the heat of reaction, ΔH, (enthalpy change) for this reaction. Determine the activation energy, E a for this reaction. Answered: Label the energy diagrams and complete… | bartleby Label the energy diagrams below and complete the statements about each. > reactants products released to stays the same Efinal reactants products decreases Efinal Einitial Efinal Einitial increases ДЕ ДЕ Energy of system Energy of system absorbed from surroundings. Energy is surroundings. Energy is Einitial Energy, E Energy, E Expert Solution Chapter 6 Cellular Respiration Quiz Flashcards | Quizlet In the diagram below, click on the area of the cell where glycolysis occurs. Cytoplasm In glycolysis, glucose is converted to -acetyl CoA. -CO2 and H2O. -citrate. -pyruvate -NAD+ and ADP. pyruvate The NET result of two molecules of glucose undergoing glycolysis is the formation of 1 NADH and 1 ATP. 2 NADH and 2 ATP. 2 NADH and 4 ATP.
Labeling an Energy Diagram Diagram | Quizlet Starting ingredients for Forward reaction. Forward Activation Energy (Ea) Energy required to break the bonds between atoms for the FORWARD reaction. Enthalpy (∆H) Heat of reaction; PE products - PE reactants. Activation Complex. Point of reaction in which all bonds between atoms are broken and atoms are free to recombine. Answered: Label the energy diagrams and complete… | bartleby Label the energy diagrams below and complete the statements about each. Einitial reactants products Efinal Einitial Efinal increases products reactants Ennal Einitial decreases Egnal Ental Efinal Emitial stays the same decreases increases ΔΕ AE decreases increases released to Energy of system Energy of system Energy is released to surroundings. Copy_of_Energy_Diagrams_Practice - Energy Diagrams Using... Using the energy diagram below, label and answer the following questions: Highlight the correct options to complete the statement below. 1. It is an endothermic / exothermic reaction because energy is released / absorbed 2. Draw a line segment that represents the potential energy of the reactants and label the line. 3. Draw a line segment that represents the potential energy of the products ... How does the energy level diagram show this reaction is exothermic? Label ΔH as positive or negative. Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases.
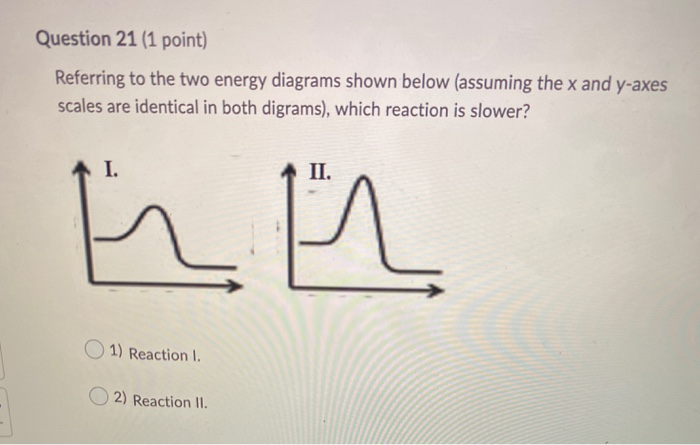
5.6: Reaction Energy Diagrams and Transition States The activation energy is what determines the kinetics of a reaction: the higher the energy hill, the slower the reaction. At the very top of the energy barrier, the reaction is at its transition state (TS), which is the point at which the bonds are in the process of breaking and forming. The transition state is an ' activated complex': a ... Solved Label the energy diagrams below and complete the - Chegg See Answer Question: Label the energy diagrams below and complete the statements about each. 6Q18 Show transcribed image text Expert Answer 91% (23 ratings) The answers to the brackets are given top-down for the given flowch … View the full answer Transcribed image text: Label the energy diagrams below and complete the statements about each. Energy Diagrams Flashcards | Quizlet Exothermic Potential Energy Diagram a chemical reaction where the Potential Energy of the product (s) is lower than that of the reactant (s). The chemical equation is going to represent energy written with the product (s) or subtracted from the reactant (s) Endothermic Potential Energy Diagram Environmental Science Chapter 5 Extra Credit Answer Key - Quizlet Drag the labels onto the flowchart to identify the sequence in which carbon moves through these organisms 1. Carbon dioxide enters a plant... 2. A primary consumer... 3. Carbon enters a higher level... 4. Fungi uses... 5. Cellular respiration... Concept Review: Biogeochemical Cycles Drag each description to the appropriate bin. Water Cycle:
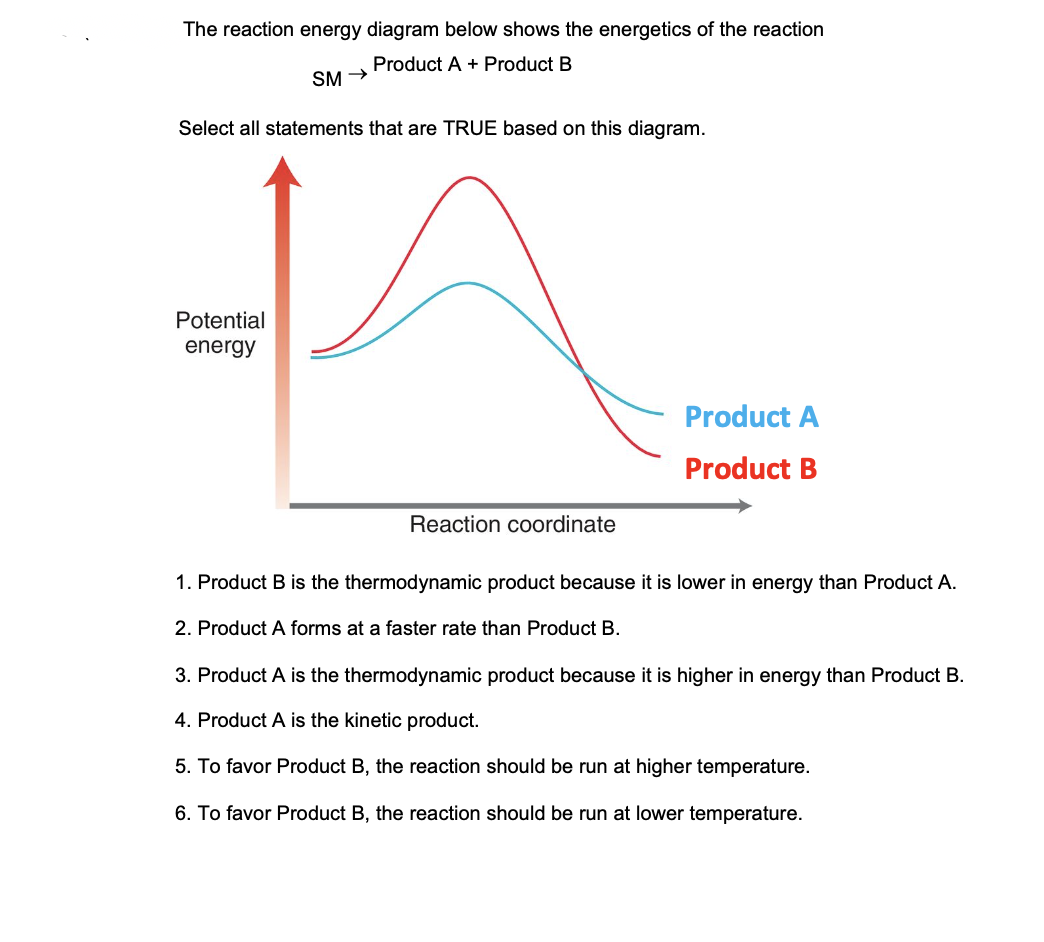
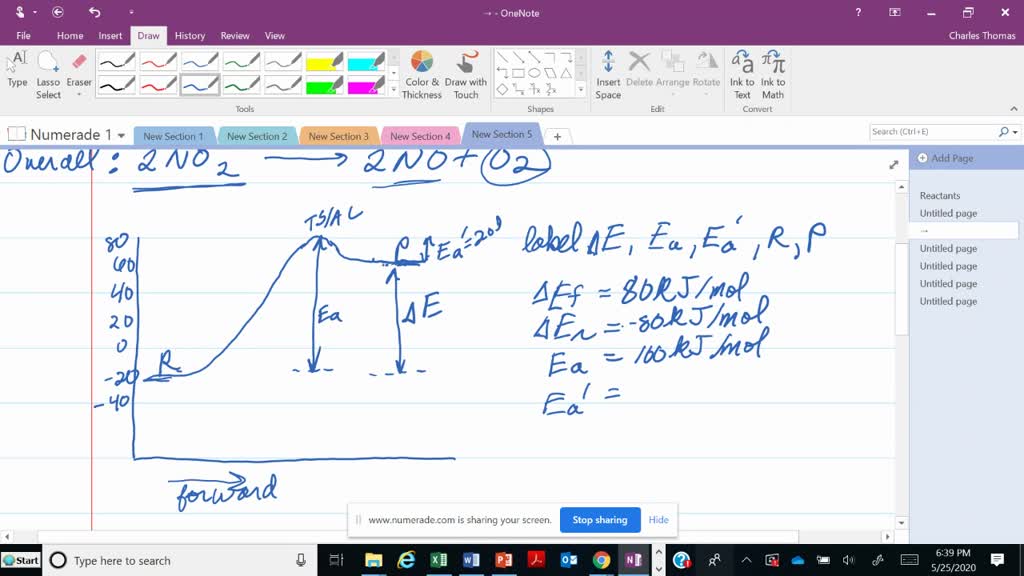
How would you draw and label energy diagrams that depict the following ... We would split up the given values in terms of thermodynamics and kinetics for the reactions. Thermodynamics DeltaE'_(fwd) = -"10 kJ/mol" DeltaE'_(rev) = -"40 kJ/mol" DeltaE_(fwd) = -"95 kJ/mol" Kinetics E_a' (fwd) = "40 kJ/mol" E_a' (rev) = "30 kJ/mol" E_a (fwd) = "20 kJ/mol" The thermodynamic values correspond to the reactants vs. products. The kinetic values correspond to the reactants vs ...
SOLVED: Label the energy diagrams below and complele the statements ... VIDEO ANSWER: There is an amount of energy present during two chemical reactions. One of the reactions is absorbed energy and the other is released energy. For the reactant and product, label the reaction for the label and energy level. To show the. Download the App!
CHM 111 Final Exam Flashcards | Quizlet Label each variable in the equation below with the property it represents. d = m = MP --- ---- V RT d = density m = mass V = volume M = molar mass P = pressure R = universal gas constant T = absolute temperature Assign each of the given labels to the flask that it correctly describes. OPTIONS: Lowest PB, Greatest Ptotal Greatest XB, Lowest Ptotal,
Energy Diagrams of Reactions | Fiveable Energy Diagrams Physical or chemical processes can be described through energy diagrams. As mentioned before, reactions can be categorized as endothermic or exothermic processes. The energy diagrams below show what should be known for the test. Image Courtesy of Pinterest Before looking at the specifics of each, you should be aware of a few terms:
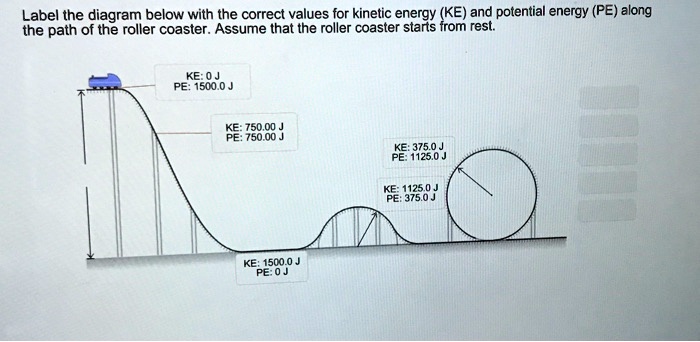
Drag each label to the correct location on the image. Each label can be ... Drag each label to the correct location on the image. Each label can be used more than once. The position-time graph describes the motion of a moving object. Describe the motion represented by each segment of the graph. not moving constant velocity positive acceleration negative acceleration
Solved Label the energy diagrams below and complete the - Chegg Label the energy diagrams below and complete the statements about each. Question: Label the energy diagrams below and complete the statements about each. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Help. Show transcribed image text Best Answer
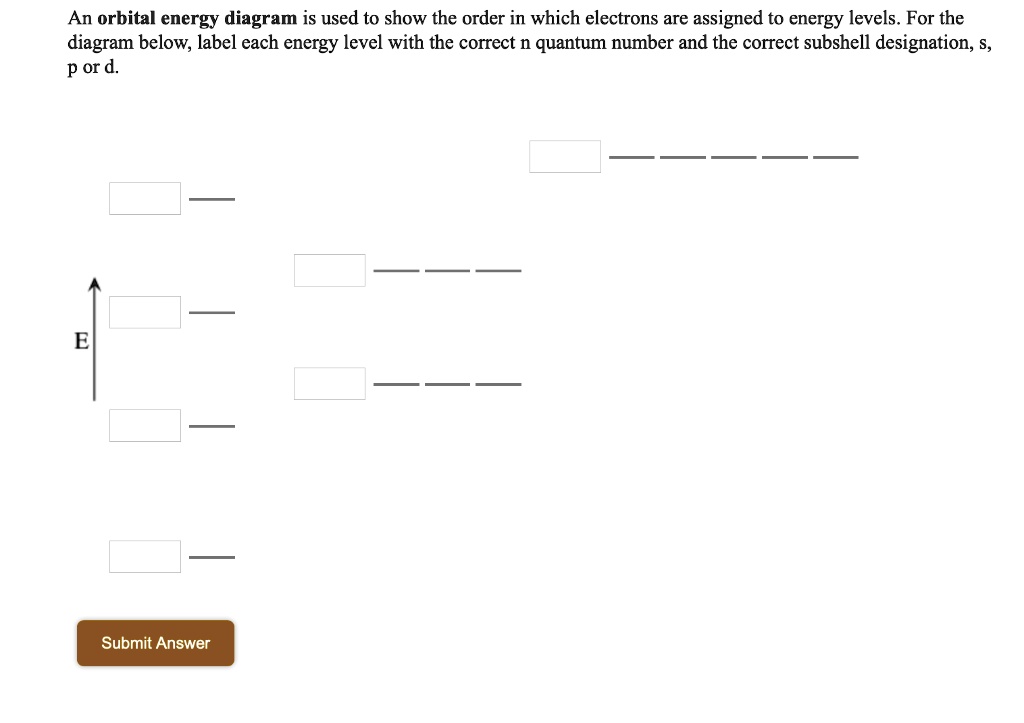
Energy Level Diagram - Different Energy Shells Around the Nucleus - BYJUS Below is a blank energy level diagram which helps you depict electrons for any specific atom. At energy level 2, there are both s and p orbitals. The 2s has lower energy when compared to 2p. The three dashes in 2p subshells represent the same energy. 4s has lower energy when compared to 3d. Therefore, the order of energy levels is as follows:
Energy Profiles (Energy Diagrams) Chemistry Tutorial - AUS-e-TUTE Energy (heat) is a product of the reaction: N 2 (g) + 3H 2 (g) → 2NH 3 (g) + 92.4 kJ mol -1. In order for energy to be conserved during the chemical reaction, the "energy of the reactants" must be greater than the "energy of the products". energy of reactants = energy of products + energy released.
18.4: Potential Energy Diagrams - Chemistry LibreTexts A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change ( Δ H) is positive for an endothermic reaction and negative for an exothermic reaction.
Label the energy diagrams below and complete the statements about each ... Label the energy diagrams below and complete the statements about each. 1. See answer. Advertisement. QueenCurlz. Where is the question? Advertisement.
6.7: Energy Diagrams - Chemistry LibreTexts In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ' reaction coordinate ', tracing from left to right the progress of the reaction from starting compounds to final products. The energy diagram for a typical one-step reaction might look like this:
Solved Label the energy diagrams below and complete the - Chegg Question: Label the energy diagrams below and complete the statements about each. Etinal reactants products increases w Einitial Energy. E Energy, E absorbed from products reactants released to Etial Entit Etinal Einti ΔΕ 0 ΔΕ 0 decreases Energy of system Energy of system Energy is ways the Energy is surroundings surroundings same Reset Zoom
Check 1 Label the energy diagrams below and complete the statements ... Check 1 Label the energy diagrams below and complete the statements about each. 3.57 points stays the same reactants products eBook References Finitial Energy, E Energy, E decreases increases products reactants Efinal Elina Einitial Ennal Einitial released to AE 0 AE 0 Energy of system Energy of system Energy is absorbed from surroundings.
Label the energy diagrams below and complete the statements about each. Label the energy diagrams below and complete the statements about each. + 20 Watch For unlimited access to Homework Help, a Homework+ subscription is required. John Edward Cayas Lv10 27 Sep 2020 Unlock all answers Get 1 free homework help answer. Already have an account? Log in Like Home Homework Help 3,800,000 Chemistry 830,000



![Answered: Based on the diagrams below, A] use the… | bartleby](https://content.bartleby.com/qna-images/question/9f1377a6-0384-45ba-92bc-919b2d05ef4c/11a50c21-158d-48d7-80af-84fe6da050e4/ldt74ym_thumbnail.png)












.png)


0 Response to "40 label the energy diagrams below and complete the statements about each"
Post a Comment