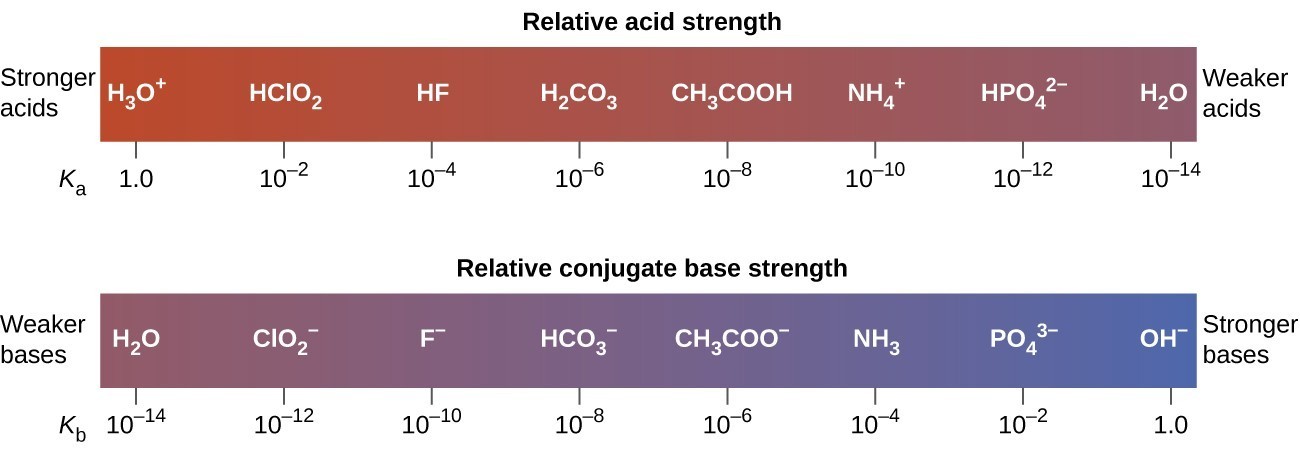
41 label ch3coo− as being a strong base, a weak base, or a species with negligible basicity.
SOLVED:Label each of the following as being a strong base, a weak base ... VIDEO ANSWER: Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the formula of its conjugate acid, and indicate whether the conjugate acid is Answered: Label each of the following as being a… | bartleby Label each of the following as being a strong base, a weak base,or a species with negligible basicity. In each case write the formulaof its conjugate acid, and indicate whether the conjugateacid is a strong acid, a weak acid, or a species with negligibleacidity: (a) CH3COO-, (b) HCO3-, (c) O2-, (d) Cl-, (e) NH3. Question
Acids and Bases Flashcards | Quizlet negligible basicity CH3COO- strong base, weak base, or a species with negligible basicity? weak base CH3COO- Conjugate acid CH3COOH Is CH3COOH a strong or weak acid? weak acid Is HCO3 a strong or weak acid or base? weak base What is the formula of HCO3- conjugate acid H2CO3

Label ch3coo− as being a strong base, a weak base, or a species with negligible basicity.
SOLVED:Label each of the following as being a strong base, a weak base ... We have to level each of the following as being a strong base a week based or species with negligible basis city. In each case we have to write the conjugate acid and we have to indicate it ... Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the formula of its conjugate ... Solved Label each of the following as being a strong acid, a - Chegg Part A: Determine whether each of the following is a strong acid, a weak acid, or a species with negligible acidity. Drag the appropriate items to their respective bins. Part B: Write the formula of the conjugate base for HNO2. Part C: Write the formula of the conjugate base for H2SO4. Part D: Write the formula of the conjugate base for HPO42−. Label each of the following as being a strong base, a weak base, or a ... Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the formula of its conjugate acid, and indicate whether the conjugate acid is a strong acid, a weak acid, or aspecies with negligible acidity: (a) CH3COO- , (b) HCO3-, (c) O2-, (d) Cl-, (e) NH3. Solution.pdf Expert's Answer
Label ch3coo− as being a strong base, a weak base, or a species with negligible basicity.. Solved Item 31 Label each of the following as being a strong | Chegg.com Science; Chemistry; Chemistry questions and answers; Item 31 Label each of the following as being a strong base, a weak or a species with noble basicly, in each case write the formula of its conjugated and indicate whether the consgate acid is a strong sold, we dorate with negible acidity Part Write the form of CH.COO conjugate sold Express your answers a chemical expression keyboard workouts ... Label each of the following as being a strong acid, a weak acid, or a ... Label each of the following as being a strong acid, a weak acid, or a species with negligible acidity. In each case write the formula of its conjugate base, ... Solved Label each of the following as being a strong base, a - Chegg a. strong base b. weak base c. species with negligible basicity 2. Write the formula of CH3COO−CH3COO− conjugate acid. Express your answer as a chemical expression. 3. Indicate whether the conjugate acid of CH3COO−CH3COO− is a strong acid, a weak acid, or a species with negligible acidity. a. strong base b. weak base SOLVED:Label each of the following as being a strong base, a weak base ... Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the formula of its conjugate acid, and indicate whether the conjugate acid is a strong acid, a weak acid, or a species with negligible acidity: ( a) C H 3 C O O −, ( b) H C O 3 −, ( c) O 2 −, ( d) C l −, ( e) N H 3 Answer
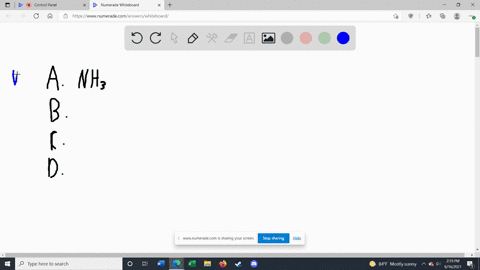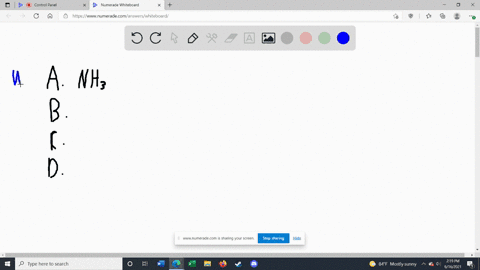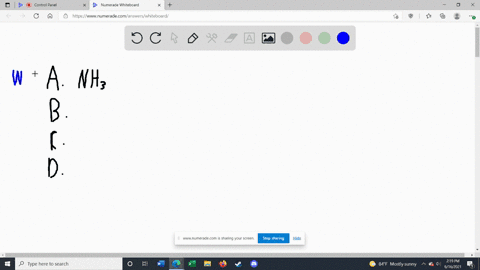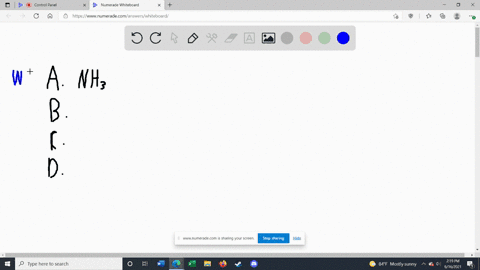
Solved Label each of the following as being a strong base, a - Chegg Transcribed image text: Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the for- mula of its conjugate acid, and indicate whether the conjugate acid is a strong acid, a weak acid, or a species with negligible acidity: (a) CH,COOT, (b) HCO, (e)O, (d) CI, (e) NH, 16.21 Solved Label each of the following as being a strong base, a - Chegg Question: Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the formula of its conjugate acid, and indicate whether the conjugate acid is a strong acid, a weak acid, or a species with negligible acidity: (a) CH3COO-, (b) HCO3-, (c) O2-, (d) Cl-, (e) NH3. [Solved] Label each of the following as being a st | SolutionInn Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the formula of its conjugate acid, and indicate whether the conjugate acid is a strong acid, a weak acid, or a species with negligible acidity: (a) CH3COO-, (b) HCO3-, (c) O2-, (d) Cl-, (e) NH3. Answer Analyze/Plan. Label each of the following as being a strong base a weak base or a ... species with negligible acidity 19. Label each of the following as being a strong base, a weak base, or a species with negligiblebasicity. In each case write the formula of its conjugate acid, and indicate whether the conjugateacid is a strong acid, a weak acid, or a species with negligible acidity.
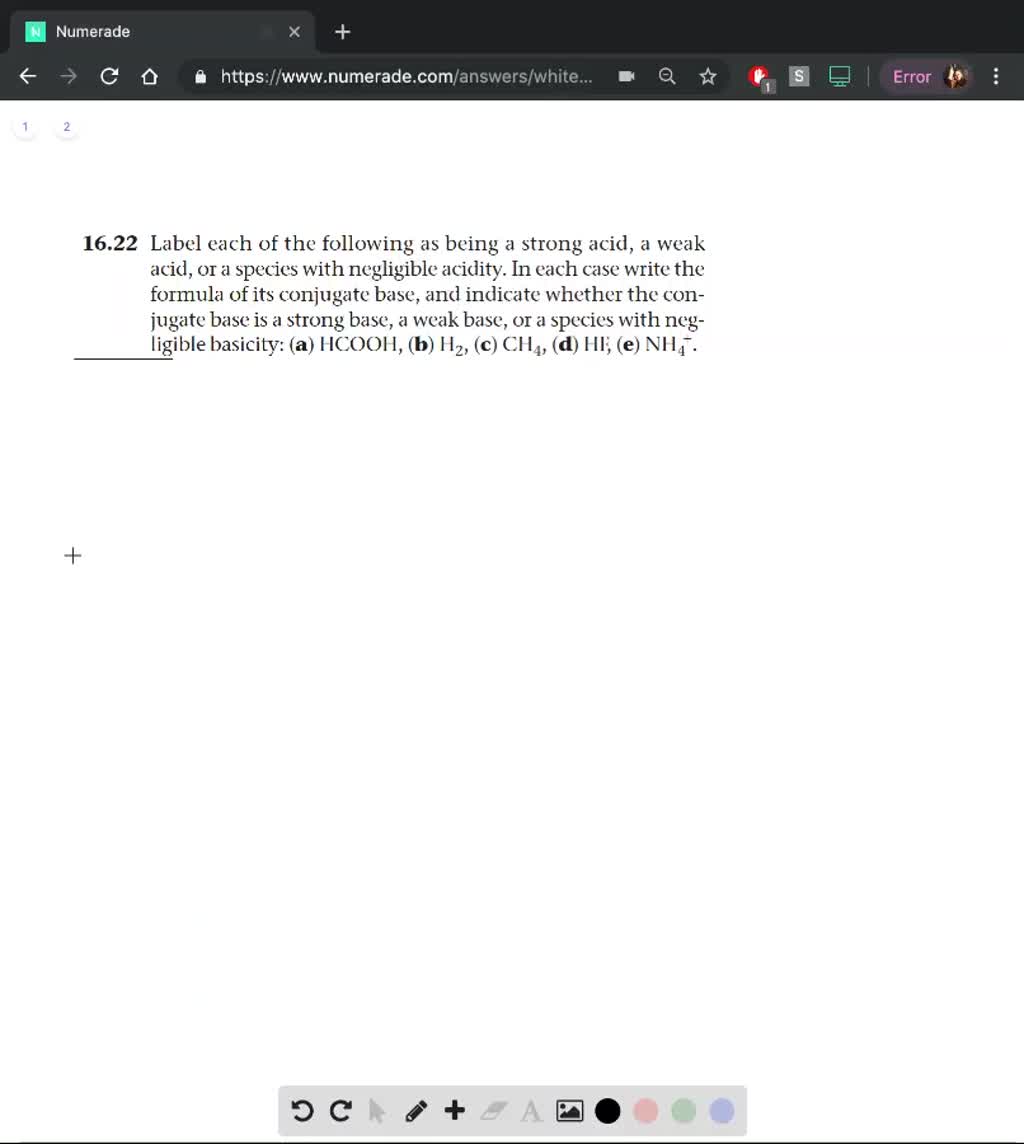
Answered: Label each of the following as being a… | bartleby ASK AN EXPERT. Science Chemistry Label each of the following as being a strong acid, a weak acid, or a species with negligible acidity. In each case write the formula of its conjugate base, and indicate whether the conjugate base is a strong base, a weak base, or a species with negligible basicity: (a) HCOOH, (b) H2, (c) CH4, (d) HF, (e) NH4 +. Is CH3COOH an acid or base? Strong vs Weak - Acetic acid - Topblogtenz A very weak base always forms a strong conjugate acid. So, CH 3 COOH is a weak acid that forms a conjugate base according to the concept of conjugate acid-base pair. The conjugate base of CH3COOH is Acetate (CH 3 COO-). Acetate is a monocarboxylic acid anion resulting from the removal of a proton from the carboxy group of acetic acid. Chapter 16: Practice Flashcards | Quizlet a weak base CH3COOH, weak acid b. strong base OH- strong base Predict the products pf the following acid-base reactions and predict if the equilibrium lies to the left or right of the reaction arrows. a. CH3COOH (aq) + HS- (aq) = b. NO2- (aq) + H20 (l) = a. CH3COO- + H2S, right b. HNO3 + OH-, left Is CH3COO a strong base because its conjugate acid is weak? Answer (1 of 2): No. It's a weak base because it's conjugate acid is weak. The pairings are Acid : Base Strong : very weak. Weak : weak Very weak : strong. Very weak means it does not act as a base or acid when you dissolve it in water. Weak means it partially reacts with water to form som...
Label each of the following as being a strong base, a weak b | Quizlet Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the formula of its conjugate acid, and indicate whether the conjugate acid is a strong acid, a weak acid, or a species with negligible acidity: CH3COO−CH_3COO^- CH3 COO− Solutions Verified Solution A Solution B Step 1 1 of 2
Label each of the following as being a strong base, a weak b | Quizlet Question Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the formula of its conjugate acid, and indicate whether the conjugate acid is a strong acid, a weak acid, or a species with negligible acidity: O2− Solutions Verified Solution A Solution B Step 1
OneClass: Label each of the following as being a strong base, a weak ... Get the detailed answer: Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the for OneClass: Label each of the following as being a strong base, a weak base, or a species with negligib...
Is formic (HCOOH) an acid or base? Weak or Strong - Topblogtenz HCOOH is considered an acid. It releases H + ions when dissolved in an aqueous solution. And acid is a substance that donates the proton to other compounds or releases H + ions in a water solution. Therefore, HCOOH is acid, since it releases H + ions in a water solution. It has a pH value of 2.38 in 0.10 M solution.
SOLVED: Label each of the following as being a strong base, a weak base ... Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the formula of its conjugate acid, and ...
J hcho2aq is the b l acid po34aq is the b l base - coursehero.com J hcho2aq is the b l acid po34aq is the b l base. School University of Houston, Clear Lake; Course Title CHEM CHEM-131; Uploaded By vivi68. Pages 7 Course Hero uses AI to attempt to automatically extract content from documents to surface to you and others so you can study better, e.g., in search results, to enrich docs, and more.
Chapter 16 Acid-Base Equilibria Flashcards | Quizlet Weak base; CH3COOH; weak acid 16.21 (b) Label if the following is a strong base, weak base or species with negligible basicty. Write the formula for the conjugate acid, and indicate whether the conjugate acid is a strong acid, weak acid, or a species with negligible acidity: HCO3^- weak base; H2CO3; weak acid
Part j label ch4 as being a strong acid a weak acid - Course Hero ANSWER : strong base weak base species with negligible basicity Correct Part M LabelCH3NH+3 (an ion related toNH+4) as being a strong acid, a weak acid, or a species withnegligible acidity. ANSWER: strong acid weak acid species with negligibleacidity ANSWER : strong acid weak acid species with negligible acidity
Label each of the following as being a strong base, a weak base, or a ... Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the formula of its conjugate acid, and indicate whether the conjugate acid is a strong acid, a weak acid, or aspecies with negligible acidity: (a) CH3COO- , (b) HCO3-, (c) O2-, (d) Cl-, (e) NH3. Solution.pdf Expert's Answer
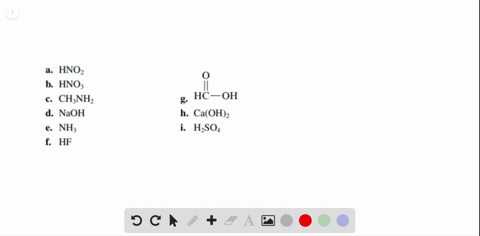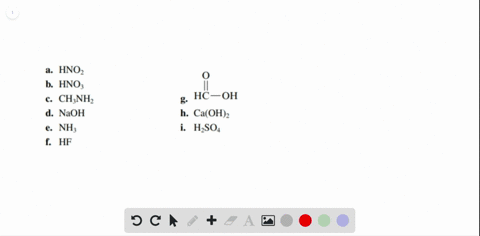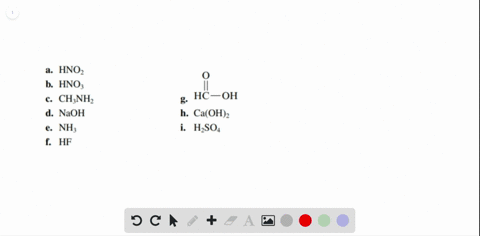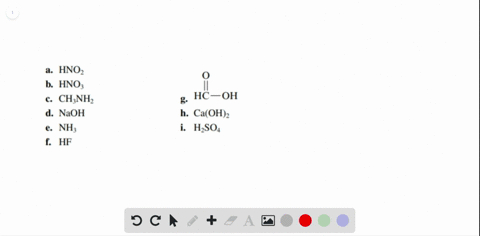
Solved Label each of the following as being a strong acid, a - Chegg Part A: Determine whether each of the following is a strong acid, a weak acid, or a species with negligible acidity. Drag the appropriate items to their respective bins. Part B: Write the formula of the conjugate base for HNO2. Part C: Write the formula of the conjugate base for H2SO4. Part D: Write the formula of the conjugate base for HPO42−.
SOLVED:Label each of the following as being a strong base, a weak base ... We have to level each of the following as being a strong base a week based or species with negligible basis city. In each case we have to write the conjugate acid and we have to indicate it ... Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the formula of its conjugate ...


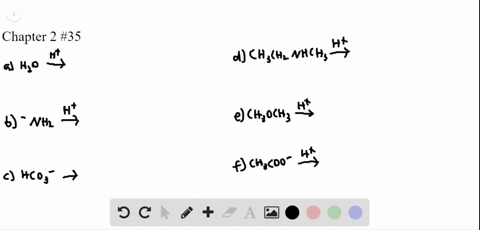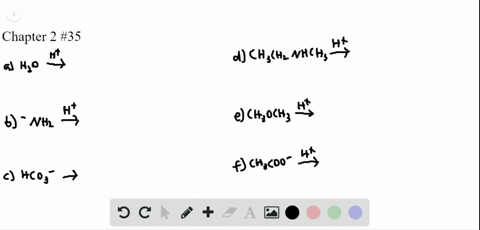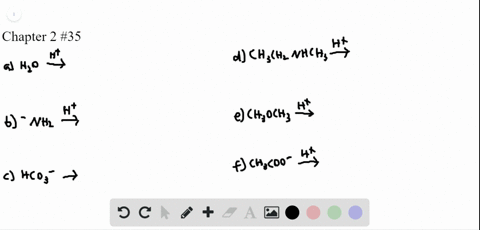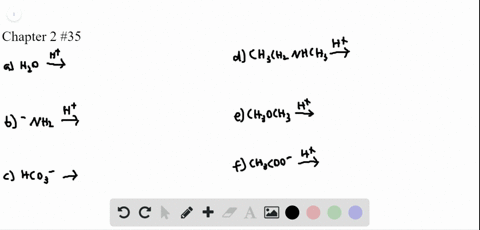












0 Response to "41 label ch3coo− as being a strong base, a weak base, or a species with negligible basicity."
Post a Comment