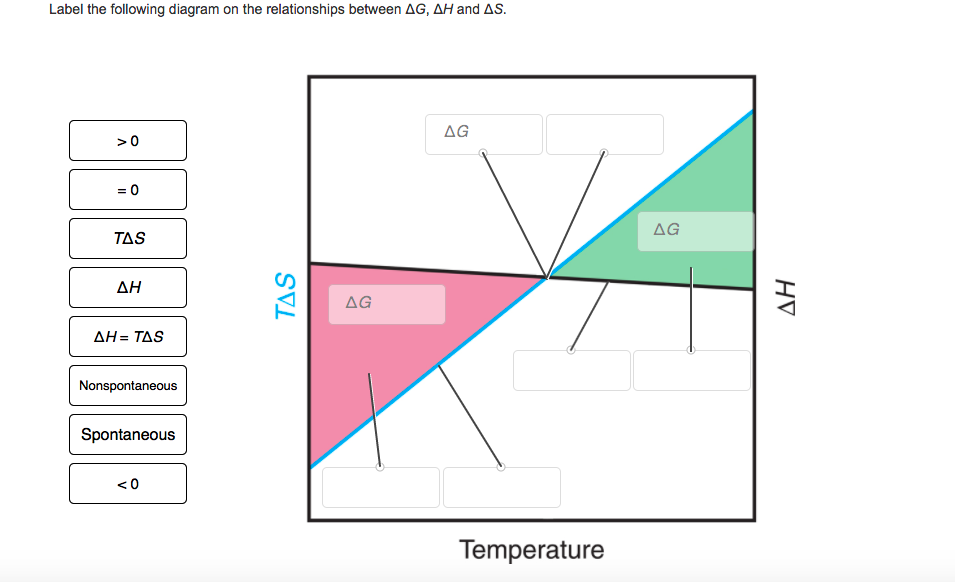
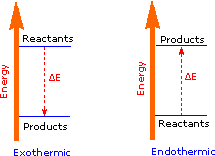
37 Label The Following Diagram On The Relationships Between δg, δh And δs.
Δ H is the enthalpy change of the system. Multiplying both sides of this equation by − T, and rearranging yields the following: −T ΔSuniv = ΔH − T ΔS − T Δ S univ = Δ H − T Δ S. Comparing this equation to the previous one for free energy change shows the following relation: ΔG = −T ΔSuniv Δ G = − T Δ S univ. The free. Nov 28, 2016 · Label the following diagram on the relationships between δg. Applying δg δh tδs the reaction is not feasible under standard pressure at any temperature because for any value of temperature gibbs free energy δg 0. Label the diagram on the relationships between g h and s this problem has been solved. 0 0 t delta s delta h delta h t delta s.
This famous relationship of free energy change to changes in enthalpy and entropy shows us the balance between entropy changes in the system and the surroundings, and how that balance depends on temperature. Note that T stands for the absolute temperature in Kelvin, so its value is always positive. Use the relationship ΔG = ΔH sys - T ΔS.
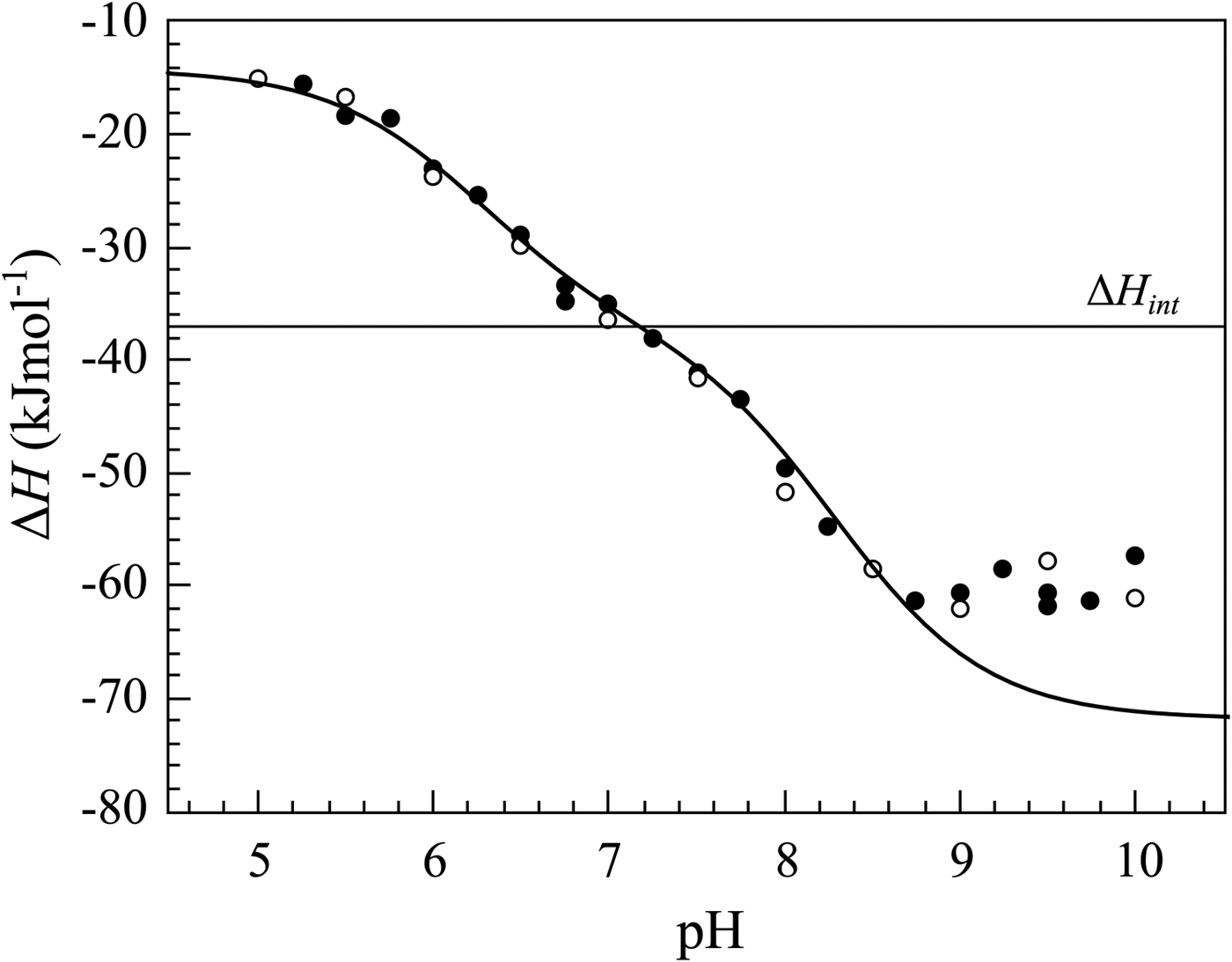
Label the following diagram on the relationships between δg, δh and δs.
Answer: http://web.mit.edu/2.813/www/readings/Ellingham_diagrams.pdf PDF An Ellingham diagram is a plot of ∆G versus temperature.. these two points, read off the... theory; hybridization; relationships between ΔG, ΔH, and ΔS; and Hess's law. These are examples of principles that you should know, but this is NOT an all-inclusive list. You are responsible for knowing the following guidelines for relative MO energies: For homonuclear diatomic molecules: • The relative E ordering is πpx and πpy < σpz. a. The diagram below at right shows the energy pathway for the reaction O3 + NO → NO2 + O2. Clearly label the following directly on the diagram. i. The activation energy (Ea) for the forward reaction ii. The enthalpy change (ΔH) for the reaction. b. The reaction 2 N2O5 → 4 NO2 + O2 is first order with respect to N2O5. i.
Label the following diagram on the relationships between δg, δh and δs.. ΔG° 1 E a1 E a2 ΔG° 2 E a3 ΔG° 3 ΔG° 130 The Hammond Postulate - an intuitive relationship between rate (E a) and product stability (ΔG°). The structure of the transition state more closely resembles the nearest stable species (i.e., the reactant, intermediate, or product). For an endothermic reaction (ΔG° > 0), the TS is nearer. Reorganization of Equation 5 yields Equation 6, which shows the linear relationship between lnK sp and 1/T: lnK sp = − @ ΔH R A @1 T A+ ΔS R [Eq. 6] Therefore, a plot of lnK sp versus 1/T yields a straight line with a slope of -ΔH/R and a y-intercept of ΔS/R. So Match. Gravity. Gibbs Free Energy. Click card to see definition 👆. Tap card to see definition 👆. ΔG=ΔH-TΔS (Get Higher Test Scores) Click again to see term 👆. Tap again to see term 👆. True or False- It matters whether all units in an equation match when solving for a particular variable. A. The wavelength of light absorbed is expected to increase to greater than 260 nm. B. The wavelength of light absorbed is expected to decrease to less than 260 nm. C. The wavelength of light absorbed is expected to remain constant at 260 nm. D. The sample is expected to behave more like DNA with intact base pairs. E.
ΔG = ΔH - T ΔS Multiple choice question. Kelvin.. Spontaneous reactions are said to have a _____. negative ΔG. Typically, what is the relationship between free energy and exergonic/endergonic reactions? Instructions. Exergonic reactions (negative ΔG) Endergonic reaction... In this common diagram of enzyme activity, which of the labels ... Predict whether the following processes are spontaneous as described, spontaneous in the reverse direction, or in equilibrium: (a) When a piece of metal heated to 150 °C is added to water at 40 °C, the water gets hotter. (b) Water at room temperature decomposes into H. 2 (g) and O. 2 (g), (c) Benzene vapor, C. 6. H. 6 (g), at a pressure of Exergonic reactions are also called spontaneous reactions, because they can occur without the addition of energy. Reactions with a positive ∆ G (∆ G > 0), on the other hand, require an input of energy and are called endergonic reactions. In this case, the products, or final state, have more free energy than the reactants, or initial state. diagram for the reaction, including transition states and intermediates. Label the activation energy of the rate-determining step, ΔH, and ΔG in the energy diagram. 10. The following molecule is Lisinopril, an ACE inhibitor used to treat high blood pressure. Label any functional groups present in this molecule. 11.
Learning Objectives. To know the relationship between free energy and the equilibrium constant. The sign of the standard free energy change ΔG° of a chemical reaction determines whether the reaction will tend to proceed in the forward or reverse direction.; Similarly, the relative signs of ΔG° and ΔS° determine whether the spontaniety of a chemical reaction will be affected by the. Solved: Label The Diagram On The Relationships Between ?G,... | Chegg . science. chemistry. chemistry questions and answers. Label The Diagram On The Relationships Between ?G, ?H And ?S. 19.7: ΔG° and K as Functions of Temperature. To know the relationship between free energy and the equilibrium constant. As was previously demonstrated, the spontaneity of a process may depend upon the temperature of the system. Phase transitions, for example, will proceed spontaneously in one direction or the other depending upon the. Jun 18, 2021 · The standard free-energy change can be calculated from the definition of free energy, if the standard enthalpy and entropy changes are known, using Equation 19.6.8: ΔG° = ΔH° − TΔS°. If ΔS° and ΔH° for a reaction have the same sign, then the sign of ΔG° depends on the relative magnitudes of the ΔH° and TΔS° terms.
d) The relationship between free energy (ΔG) and enthalpy (ΔH) & entropy (ΔS) for a chemical reaction at a specific temperature (T) is: ΔG = ΔH - T·ΔS Combine this with the equation from part (c) to find an equation in y = mx + b form, where the slope (m) is (-ΔH°/R). Box your answer. 2 points:
In this diagram, the activation energy is signified by the hump in the reaction pathway and is labeled. At the peak of the activation energy hump, the reactants are in the transition state, halfway between being reactants and forming products. This state is also known as an activated complex. Effect of a Catalyst.
1) 6 pts Calculate ΔG˚ at 125˚C for the following reaction. Assume that ΔH˚ and ΔS˚ are temperature independent. 2CH 3 OH(g) +3O 2 (g) 2CO 2 (g) + 4H 2 O(l) 2) 6 pts What current is required to produce 2.5 g of chromium metal from chromium (VI) oxide in 12 h? 3) 6 pts Calculate E cell for the following at 25˚C
determine the signs of ΔG and ΔS. Then state whether the starting material or product is favored at equilibrium. Classify each reaction as addition, substitution, or elimination. (a)Note: C=C bond energy = 614 Answer: Bonds broken: C-C, H-Br Bonds formed: C-H, C-Br Because ΔH & ΔG are similar in value, ΔG is also positive.
CH 142 Spring 2012 4 2. For the following voltaic cell Pb !Pb+2 (1 M) !! Cu+2 (1 M) !Cu for which the following data were obtained, determine ΔG at each temperature, then graph ΔG versus absolute T. Find the ΔH value for this battery and the ΔS value using the graph created. Note that while there can be several values for ΔG there is just one value for ΔH and
a. The diagram below at right shows the energy pathway for the reaction O3 + NO → NO2 + O2. Clearly label the following directly on the diagram. i. The activation energy (Ea) for the forward reaction ii. The enthalpy change (ΔH) for the reaction. b. The reaction 2 N2O5 → 4 NO2 + O2 is first order with respect to N2O5. i.
Energy/Reaction Coordinate! Diagrams! Thermodynamics, Kinetics ! Dr. Ron Rusay A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change (ΔGo) Enthalphy (ΔHo): the heat given off or absorbed during a reaction Entropy (ΔSo): a measure of freedom of motion ΔGo = ΔHo - TΔSo ΔG,ΔH,ΔS, ΔE are state.
Draw an energy diagram for each reaction. Label the axes, the starting material, product, transition state, ΔH o, and E a. a.) a concerted reaction with ΔH o = −80 kJ/mol and E a = 16 kJ/mol b.) a two-step reaction, A → B → C, in which the relative energy of the compounds is A < C < B, and the step A → B is rate-determining
disorder). The relationship between the enthalpy change of a reaction, ΔH reaction and the entropy change of the surroundings, ΔS surroundings is given by; ΔS surroundings = ΔH reaction T If TΔS total is equal to the Gibbs free energy change, ΔG, use the facts above to derive the equation; ΔG = ΔH reaction TΔS system (3 marks) 2.
Find the ΔH of the following reaction: C(s, gr) + O 2 (g) ---> CO 2 (g). Solution: 1) Analyze what must happen to each equation: a) first eq ---> flip it (this put the CO 2 on the right-hand side, where we want it). b) second eq ---> do not flip it, divide through by two (no flip because we need to cancel the SrO, divide by two because we only need to cancel one SrO)
So change in Gibbs free energy is equal to the change in enthalpy minus the product of temperature and entropy change of the system. ΔG = ΔH – Δ (TS) If the reaction is carried out under constant temperature {ΔT=O} ΔG = ΔH – TΔS. This equation is called the Gibbs Helmholtz equation. ΔG > 0; the reaction is non-spontaneous and.
Standard Enthalpy of Formation. Standard Enthalpy of Reaction (ΔH rxn) is the amount of heat absorbed (+ΔH value) or released (-ΔH value) that results from a chemical reaction.. ΔH rxn is calculated using the standard enthalpy of formation for each compound or molecule in the reaction. The enthalpies of all reactants are added and the sum of the enthalpies of the reactants are subtracted.
theory; hybridization; relationships between ΔG, ΔH, and ΔS; and Hess's law. These are examples of principles that you should know, but this is NOT an all-inclusive list. You are responsible for knowing the following guidelines for relative MO energies: For homonuclear diatomic molecules: • The relative E ordering is πpx and πpy < σpz.
Relationship between Rate and Energy of Activation! Referring back to our energy diagram the rate can be related! to the energy of activation (E a)! k r = Ae(-Ea/RT)! A is the Arrhenius “preexponential” factor! E a is the minimum kinetic energy required to cause the reaction to proceed! As a general guide, the rate of a reaction generally !
Free energy is also dependent on temperature. (Recall the relationship between temperature and entropy) The value "T" is given in degrees Kelvin. (To convert Centigrade to Kelvin, add 273.) The relationship between these terms is expressed by the free energy equation: ΔG = ΔH - T(ΔS)
Question: Label The Diagram On The Relationships Between ?G, ?H And ? Label The Following Diagram On The Relationships Between ?GAH And ? Nonspontaneous ?? Spontaneous Spontaneous «? ?? Nonspontaneous TAS S 0 Temperature Reset Zoom.
Answer: http://web.mit.edu/2.813/www/readings/Ellingham_diagrams.pdf PDF An Ellingham diagram is a plot of ∆G versus temperature.. these two points, read off the...
ΔG = ΔH - TΔS. The change in Gibbs free energy (ΔG) for a system depends upon the change in enthalpy (ΔH) and the change in entropy (ΔS) according to the following equation: ΔG = ΔH - TΔS. ΔGo = ΔHo - TΔSo. The relationship holds true under standard conditions or under non-standard conditions. We can take away a few generalizations.
For the reaction below δg 330 kj δh 922 kj and δs 1987 jk. Which of the following reactions will have the smallest equilibrium constant. 2 nh3g n2g 3 h2g. Answer to the diagram below represents a spontaneous reaction deltag degree 0. δh δg tδs this means that δh δg. Drag the labels to the correct bins.
Team Number: _____ 4 4 b) Write the K sp value in terms of potassium nitrate concentration ([KNO 3]). K sp = _____ Calculations c) The relationship between free energy (ΔG) and the equilibrium constant (K) for a chemical reaction at a specific temperature (T) is: ΔG = -R·T·ln K Use this equation, along with your data, to fill out the table below.




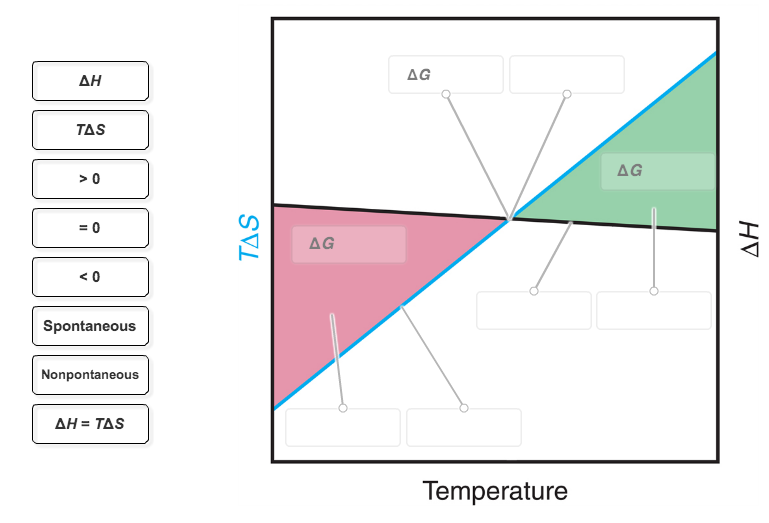


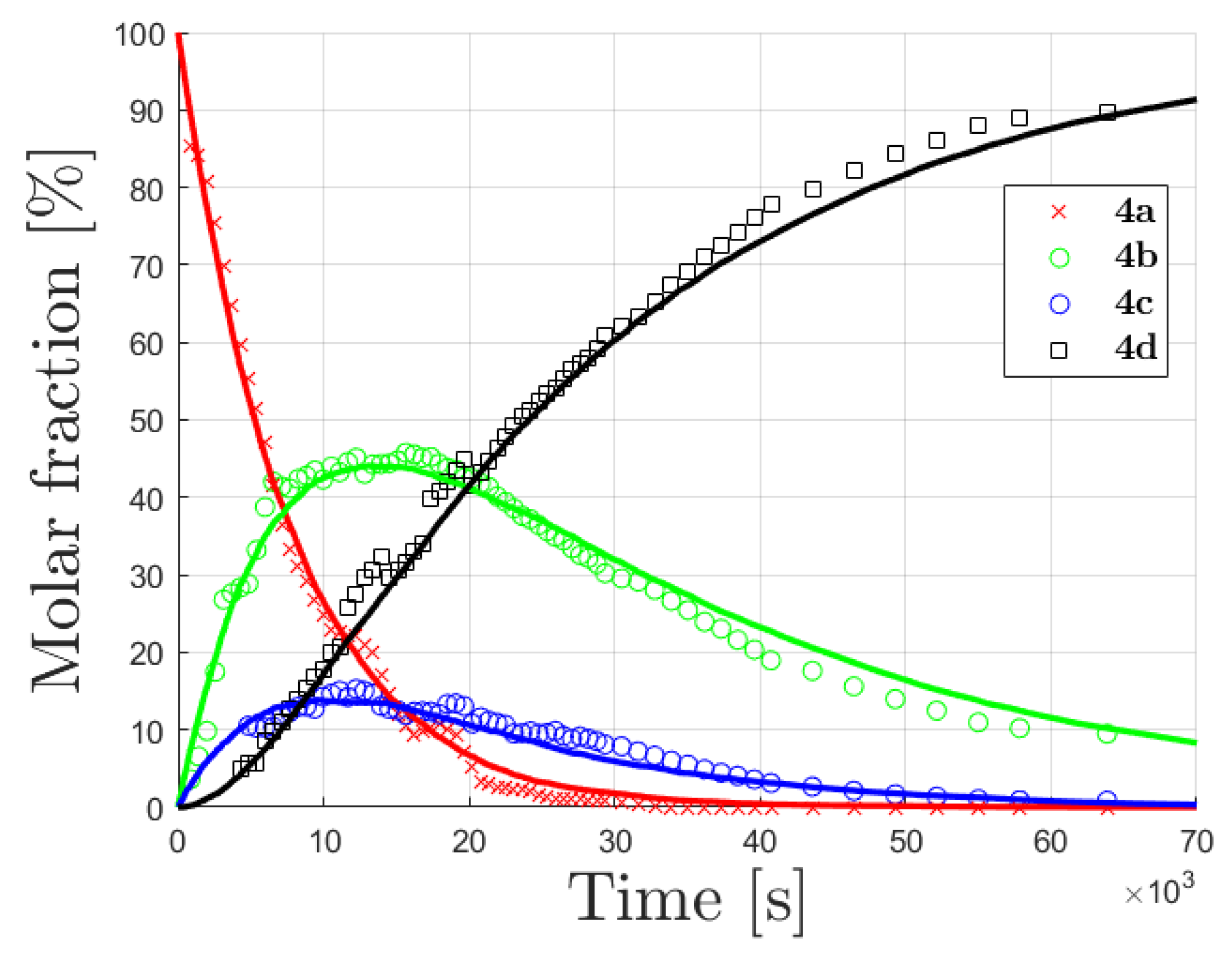







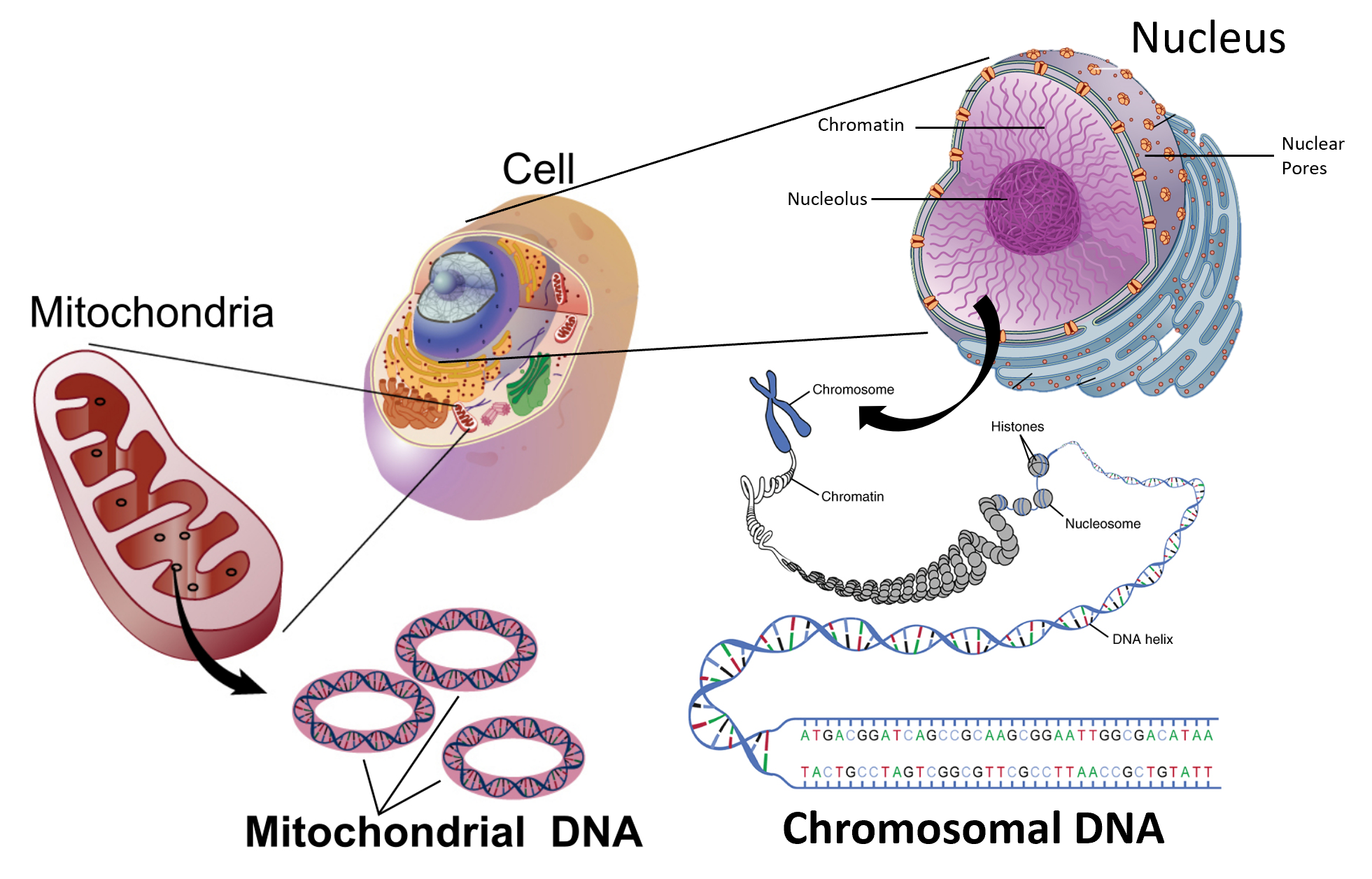




0 Response to "37 Label The Following Diagram On The Relationships Between δg, δh And δs."
Post a Comment