41 prescription drug label requirements
en.wikipedia.org › wiki › Standard_for_the_UniformStandard for the Uniform Scheduling of Medicines and Poisons Situations that may require an authority include where the drug may only have benefit in limited conditions, the true cost of the drug is high, or when there is a risk of dependence. Some states have subsets of Schedule 4 with additional requirements (see below). Schedule 4 medicines cannot be advertised directly to the public. Examples: Regulations for Flying With Prescription Drugs | USA Today In most cases, TSA doesn't allow travelers to pack liquids in containers larger than 3.4 ounces in their carry-on luggage, per the 3-1-1 liquids rule. However, medically necessary liquids are ...
PDF Menu of State Prescription Drug Identification Laws The United States is in the midst of an unprecedented epidemic of prescription drug overdose deaths.1 More than 38,000 people died of drug overdoses in 2010, and most of these deaths (22,134) were caused by overdoses involving prescription drugs.2 Three-quarters of prescription drug overdose deaths in 2010 (16,651) involved a

Prescription drug label requirements
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (1) Prescription drug labeling described in § 201.100 (d) must contain the specific information required under § 201.57 (a), (b), and (c) under the following headings and subheadings and in the... CFR - Code of Federal Regulations Title 21 - Food and Drug Administration § 201.80 - Specific requirements on content and format of labeling for human prescription drug and biological products; older drugs not described in 201.56 (b) (1). Subpart D - Exemptions From... How to Label Prescription Medications | Study.com The label should be neat, organized, and large enough print for patient's to be able to read the information. Important information should be highlighted or bolded to alert the patient to that...
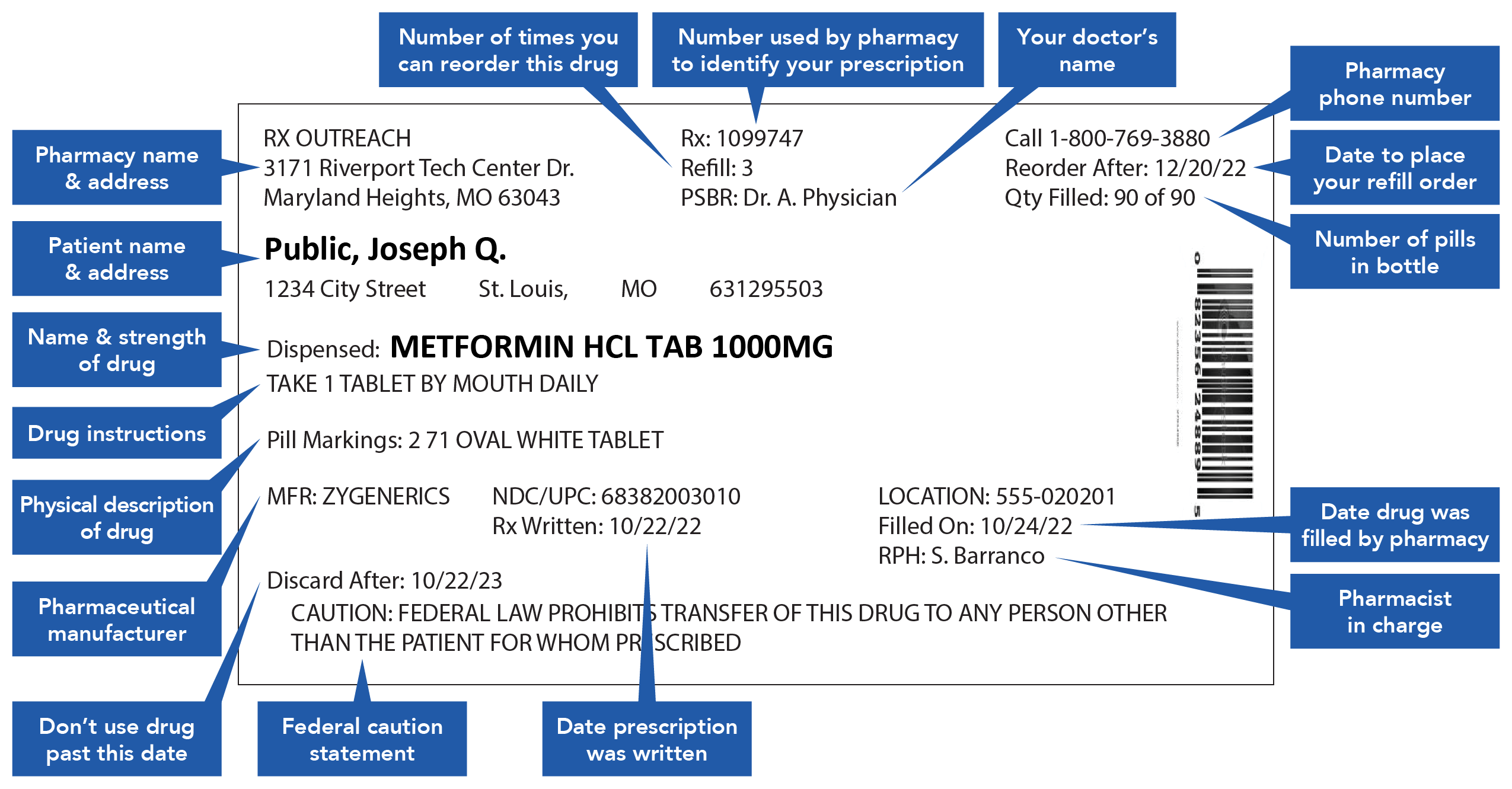
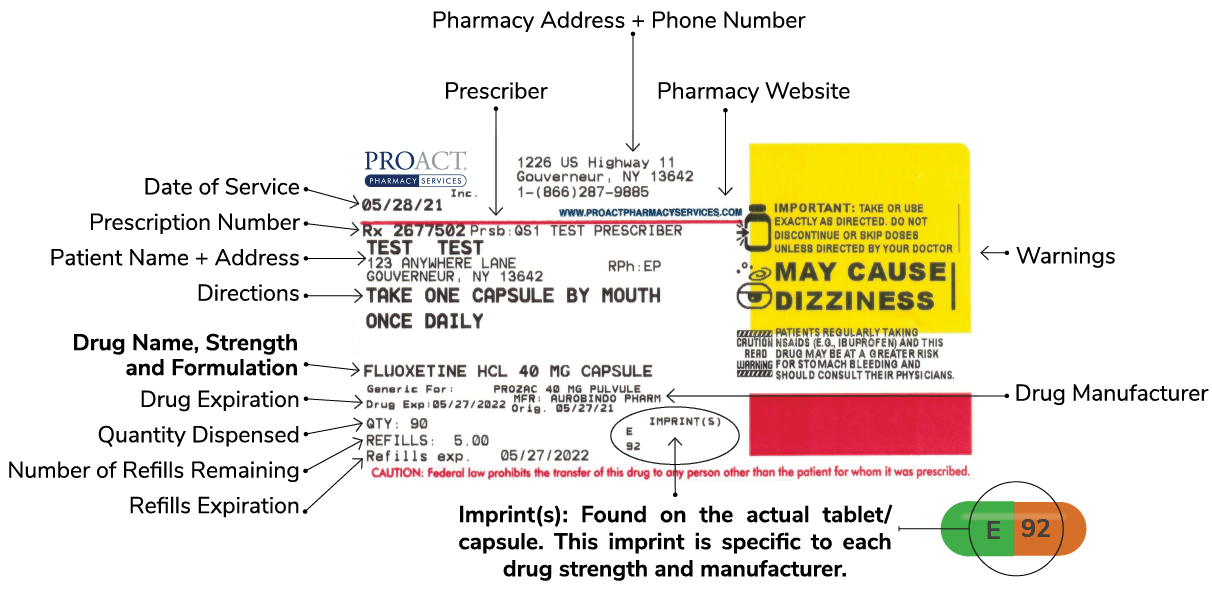
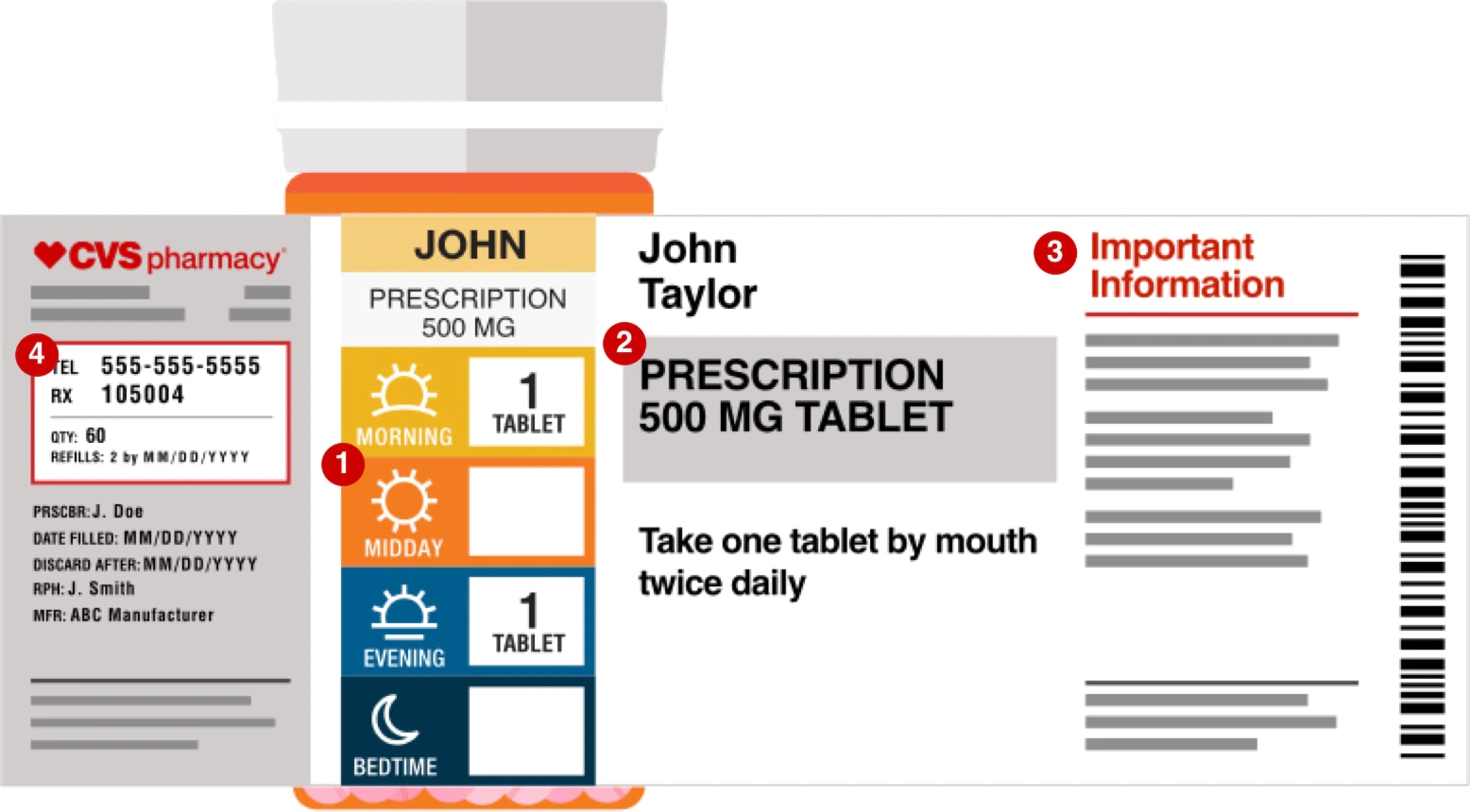
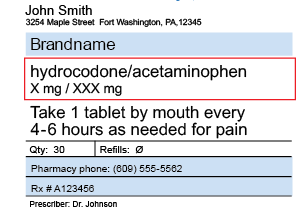
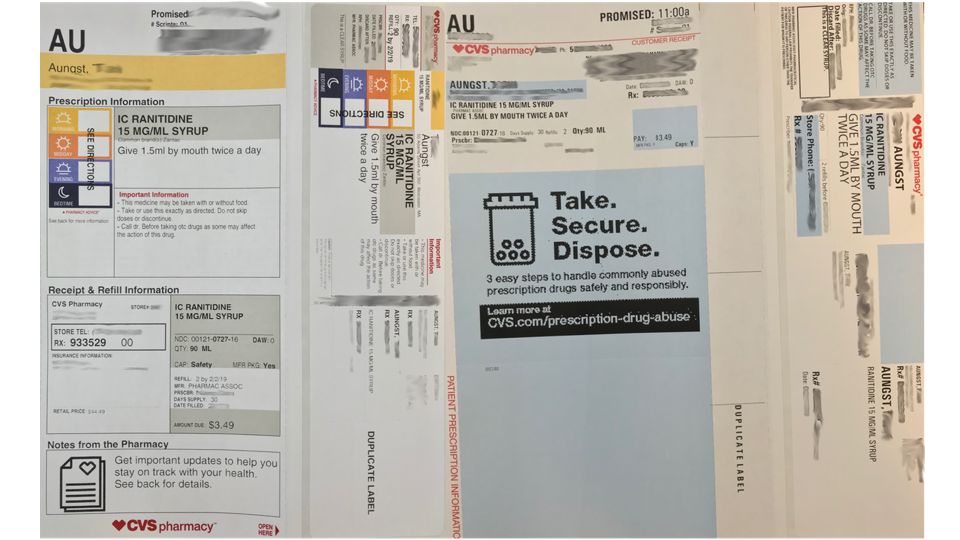
Prescription drug label requirements. Carton and Container Labeling Resources | FDA - U.S. Food and Drug ... Section 201 (k) and (m) of FD&C Act: Statutory definition of "label" and "labeling", respectively. 21 CFR 201.100 (b): Prescription label requirements. 21 CFR 201.10 (i): Small label requirements. 21 CFR 201.6: Misleading statement. 21 CFR 201.15: Prominence of required label statements. 21 CFR 610.60: Container label for biological ... NCBOP - Pharmacist FAQs The following information must be on every prescription label: 1. Name and address of the dispensing pharmacy. 2. Serial number of the prescription. 3. Date of the prescription. 4. Name of the prescriber. 5. Name of the patient. 6. Name and strength of the drug. 7. Australian Regulatory Guidelines for Prescription Medicines (ARGPM ... The Australian Regulatory Guidelines for Prescription Medicines (ARGPM) assist applicants and sponsors to register new prescription medicines or vary existing registrations in Australia. Sponsors may now apply to register a prescription medicine under the provisional approval pathway , priority review pathway or the standard prescription ... Questions and Answers: Plain Language Labelling Regulations for ... (a) The Labels and Packages Certification Form for Prescription Drugs (b) Inner/Outer Label Mock-ups Mock-ups of the inner/outer label (s) should be bilingual, in full colour, actual size and editable (e.g., PDF) (c) Package Insert Mock-Ups (d) Product Monograph/Prescribing Information
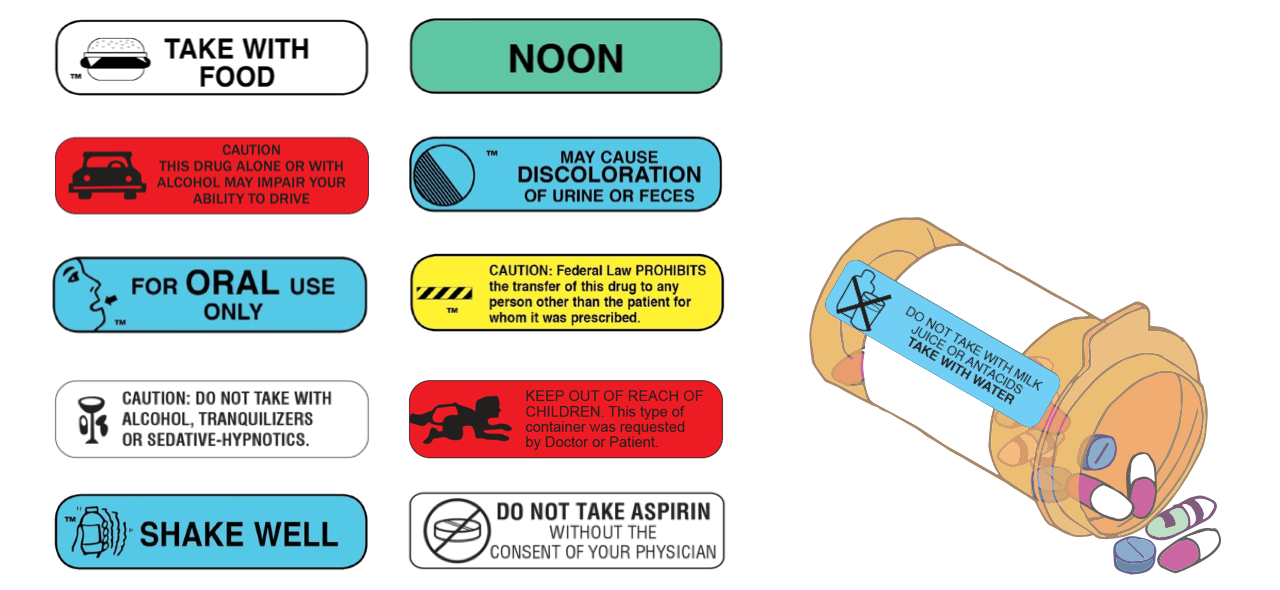
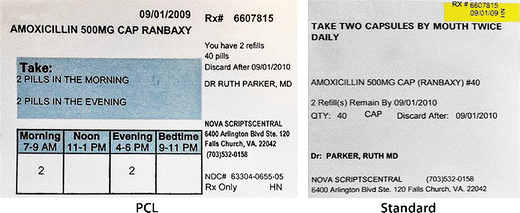
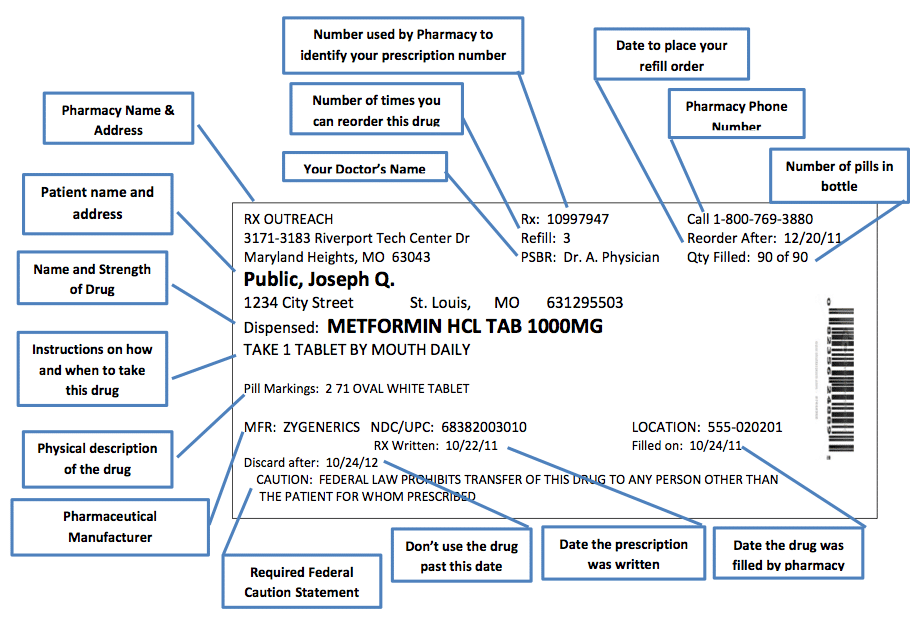
FDA Issues New RX Label Requirements - The Recovery Village Drug and ... A prescription drug label must include certain information. FDA prescription labeling requirements must be clearly printed with: Pharmacy information Doctor information Instructions Physical description of the drug Federal caution statement Dates Pharmacy prescription number Number of pills Number of times the drug can be reordered › medical-devices › general-deviceLabeling Requirements - Over-The-Counter (Non-Prescription ... FDA Regulations and requirements for labels and other written, printed, or graphic materials (labeling) that accompanies or is associated with an over-the-counter (Non-Prescription) medical device. Can you pack your meds in a pill case and more questions answered TSA does not require passengers to have medications in prescription bottles, but states have individual laws regarding the labeling of prescription medication with which passengers need to comply. Medication is usually screened by X-ray; however, if a passenger does not want a medication X-rayed, he or she may ask for a visual inspection instead. FDA's Labeling Resources for Human Prescription Drugs | FDA Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective use of the drug; and (2) includes the Prescribing Information,...
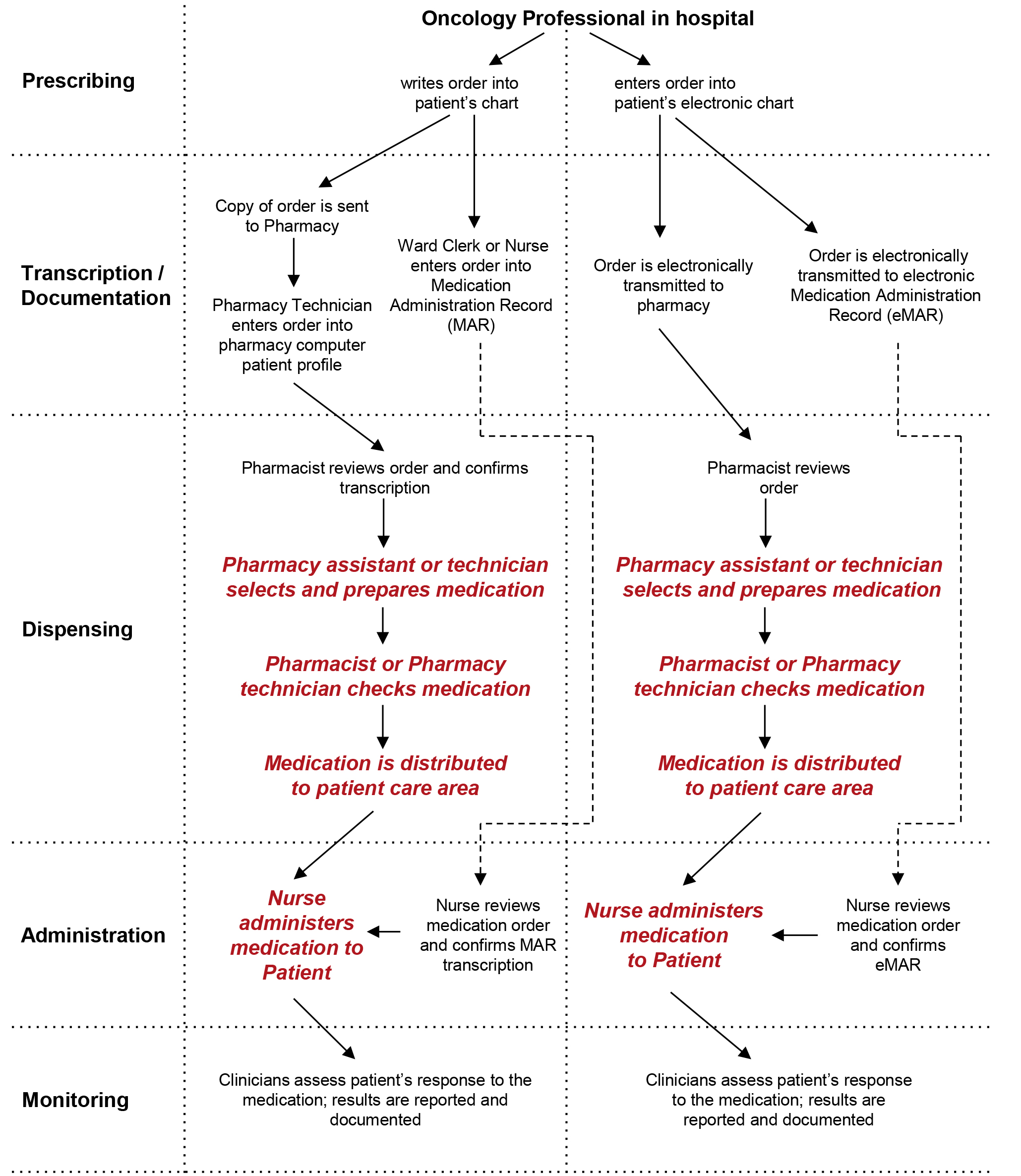
› documents › 2013/09/24Federal Register :: Unique Device Identification System Sep 24, 2013 · Another comment also recommended that the date of manufacture should be used “to determine compliance with the UDI requirements” and stated this was the approach FDA used in implementing FDA's final rule, “Bar Code Label Requirements for Human Drug Products and Biological Products” (69 FR 9120; February 26, 2004). These comments were ... Labeling guidelines for sample prescription drugs | Mass.gov Labeling requirements Practitioners must label all sample medications dispensed to patients, including those provided as part of an indigent patient drug program (see M.G.L. c. 94C §22 and 105 CMR 700.010). Labels must contain the information described below; however, the method of labeling the medications may vary. A Guide To Veterinary Prescription Label Requirements Although the size of a veterinary prescription label is small, approximately 2" x 3", the information the FDA requires is extensive. Whether the information is preprinted or computer generated, the label should contain seven distinct categories ranging from the veterinary practice name and contact info to directions on how to use the medication. eCFR :: 21 CFR Part 201 -- Labeling The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented according to the schedule specified in § 201.56(c), except for the requirement in paragraph (c)(18) of this section to reprint any FDA-approved patient labeling at the end of prescription drug labeling or accompany the ...
Types of FDA Drug Labeling and Their Requirements - PDG Medication Guides contain "prescription drug information for certain medications that pose a serious and significant public health concern, developed by the manufacturer, approved by FDA, and required to be distributed to consumers each time the medication is dispensed". [15]
Requirements on Content and Format of Labeling for Human Prescription ... The final rule also revises current regulations for prescription drug labeling of older products by clarifying certain requirements. These changes will make the labeling for older products more informative for health care practitioners. DATES: This rule is effective June 30, 2006. See section III of this document for the implementation dates of ...
USP 800 Labeling Requirements | United Ad Label Further, labeling should be done according to state and federal regulations and include the: Generic or chemical names of the active ingredients; Strength or quantity; Pharmacy lot number; Beyond-use date; Any special storage requirements; Typically, organizations use pharmacy and prescription medication labels for compounding applications.
Drug labelling - Wikipedia In line with local legislations, a pharmaceutical product should fulfill several labelling requirements for the purpose of registration: the product name, the name and quantity of each active ingredient, the name and address of the manufacture, Hong Kong registration number, batch number, expiry date and storage instructions, if any. [9]
eCFR :: 21 CFR 201.56 -- Requirements on content and format of labeling ... Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: ( 1) The labeling must contain a summary of the essential scientific information needed for the safe and effective use of the drug.
Drug Labeling Requirements of the Food and Drug Administration Governing Regulation for Drug Labelling Requirements. The primary sources of information for consumers are the labels and labeling materials, as they provide useful information such as those dealing with the safe and effective use of a drug product (e.g. indication(s), pharmacologic class and dosage), and information dealing with quality (e.g. manufacturing and expiration dates, registration ...
Pharmaceutical Labeling 101: FDA Regulations Guide The FDA has a strict code when it comes to medicine labeling and all manufacturers, big or small, must adhere to these rules to get their product FDA approved. All human prescription drugs and biological products should follow the guidelines available in 21 CFR 201.56 (d) and 201.57.
Guidance Document: Labelling of Pharmaceutical Drugs for Human Use 3.4.5 Scheduling Symbols or Pr for Drugs Containing An Ingredient Listed in the Prescription Drug List 3.4.6 Drug Identification Number 3.5 Any Panel 3.5.1 Name and Address of Manufacturer/Sponsor 3.5.2 Lot Number 3.5.3 Expiration Date 3.5.4 Adequate Directions for Use 3.5.4.1 Storage Conditions 3.5.4.2 Limit Dose Drug Products
Medicines: packaging, labelling and patient information leaflets Labels must be clear. Healthcare professionals and patients must easily be able to identify the medicine by the label. You should use the letters CD in an inverted triangle if your product is a...
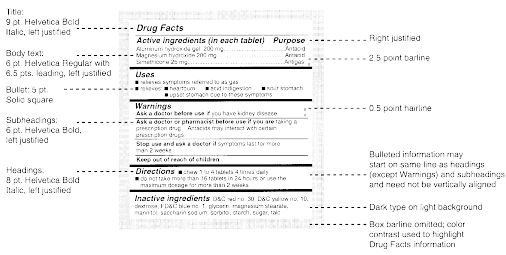
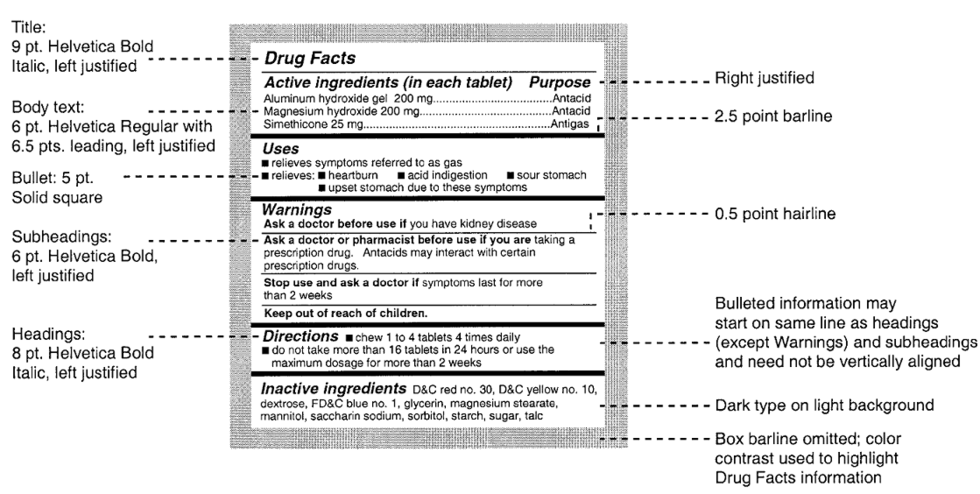
Pharmaceutical Labeling: Requirements & Guidelines To meet today's FDA regulations, labeling information on drugs must include the following in this order: - Product Name - Drug Facts Table - Active Ingredients - Purpose and Use - Warnings - Directions - Allergic Reactions - Inactive Ingredients
Labeling Information | Drug Products | FDA For prescription drug labeling resources (e.g., Prescribing Information, FDA-approved patient labeling, and carton and container labeling), please see the Prescription Drug Labeling Resources web ...
› media › 71836Guidance for Industry - Food and Drug Administration Office of Communications Division of Drug Information, WO51, Room 2201 10903 New Hampshire Ave. Silver Spring, MD 20993 Phone: 301-796-3400; Fax: 301-847-8714
› regulatory-information › search-fdaCPG Sec. 450.500 Tamper-Resistant Packaging Requirements for ... Tamper-Resistant Packaging Requirements for Certain Over-the-Counter Human Drug Products FDA Guidance. ... Direct printing of the label on the container (e.g., lithographing), is preferred to ...
PDF Chapter 61-04-06 Prescription Label Requirements 61-04-06-01. The prescription label. Controlled drugs and noncontrolled drugs dispensed pursuant to a prescription must bear a label, permanently affixed to the immediate container in which the drug is dispensed or delivered and which is received by the purchaser or patient, which must include the following: 1. The name and address of the ...
› research › healthState Prescription Drug Repository Programs Oct 01, 2021 · New Hampshire passed legislation to allow manufacturer's samples to be donated as an 'unused prescription drug' to the preexisting NH program. New Mexico passed legislation allowing for a prescription drug donation program by enacting a new section of the New Mexico Drug, Device and Cosmetic Act. This new section provided standards and ...
Prescription Label Information, Translations, and Sample Labels ... Prescription Label Information, Translations, and Sample Labels. Translations of Pill Directions ; Patient-Centered Prescription Drug Container Label Samples; Prescription Drugs: Labeling Requirements - Report to the Legislature; Statutory Requirements (4076.5) and Regulation Requirements (1707.5)
› otc-drug-facts-labelOTC Drug Facts Label | FDA This regulation required most OTC drug products to comply with the new format and content requirements by May 2002. Manufacturers may continue to use old-format labels until their inventories are ...
How to Label Prescription Medications | Study.com The label should be neat, organized, and large enough print for patient's to be able to read the information. Important information should be highlighted or bolded to alert the patient to that...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration § 201.80 - Specific requirements on content and format of labeling for human prescription drug and biological products; older drugs not described in 201.56 (b) (1). Subpart D - Exemptions From...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (1) Prescription drug labeling described in § 201.100 (d) must contain the specific information required under § 201.57 (a), (b), and (c) under the following headings and subheadings and in the...






















0 Response to "41 prescription drug label requirements"
Post a Comment