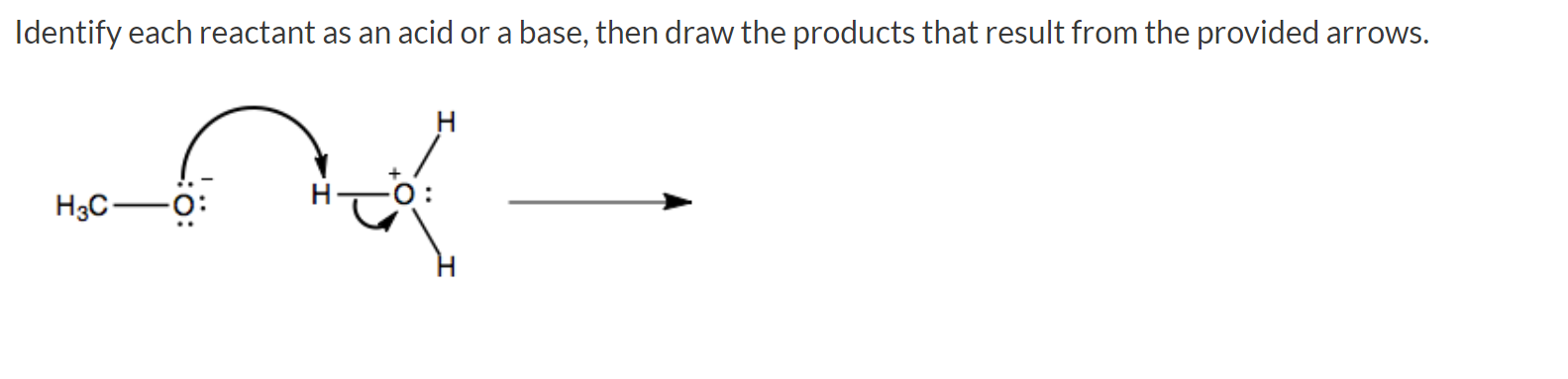
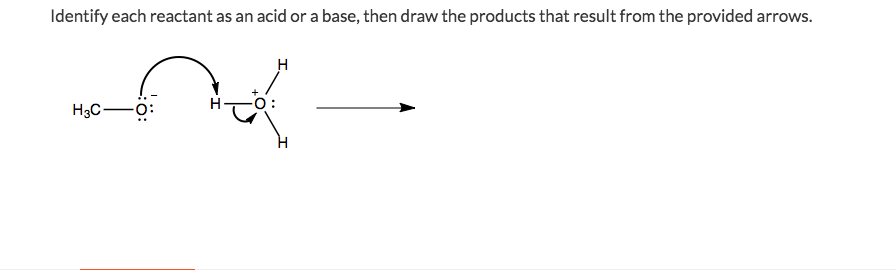
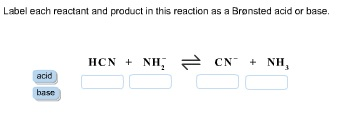
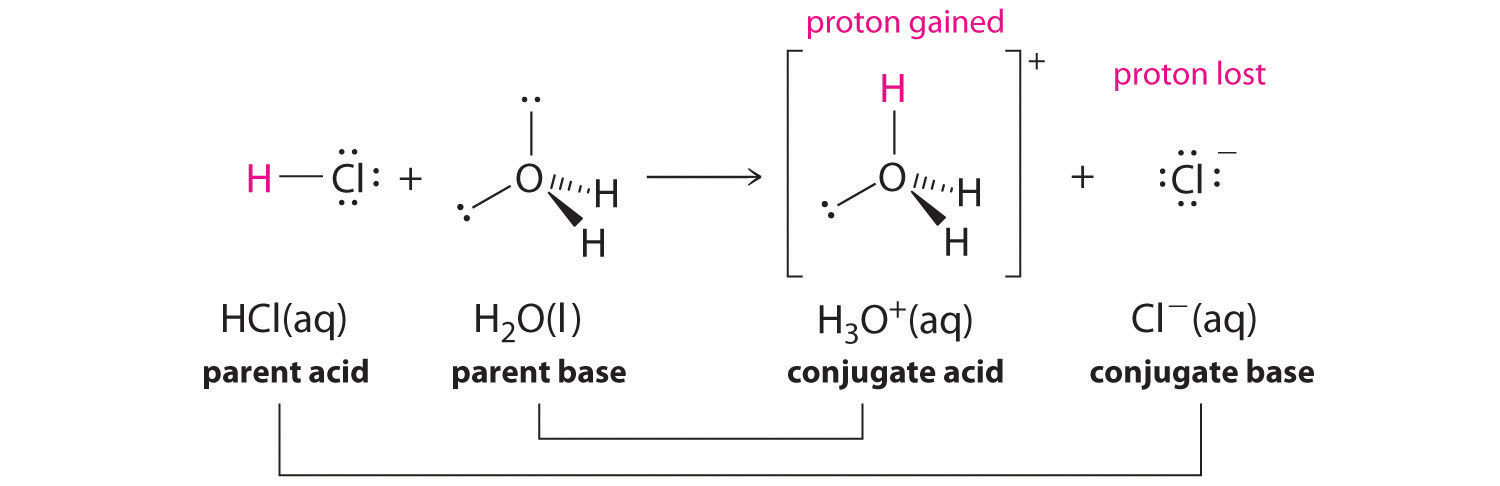
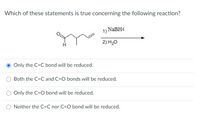
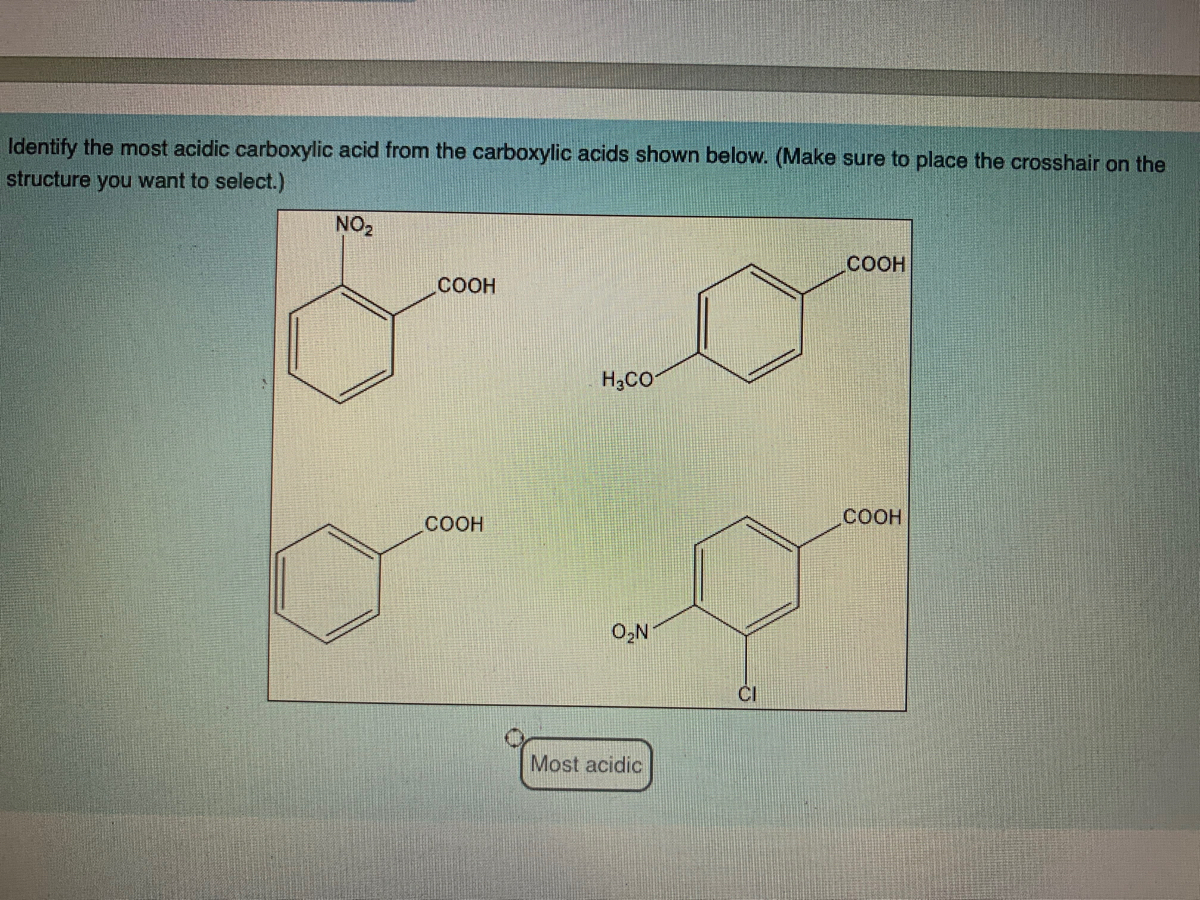
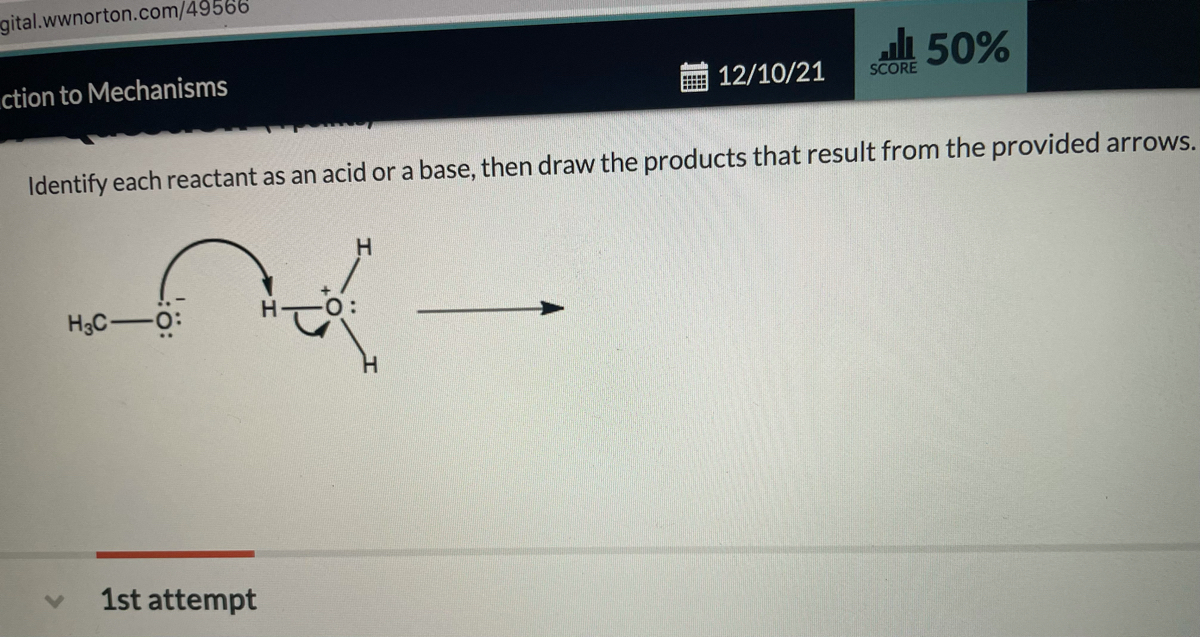
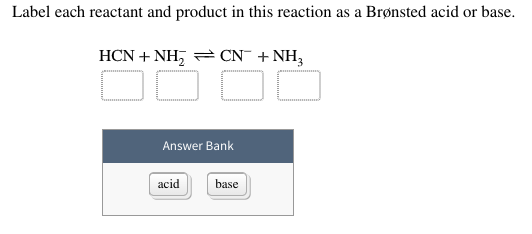
45 label each reactant and product in this reaction as a brønsted acid or base h2y
FORM 4 CHEMISTRY NOTES HANDBOOK - Educationnewshub.co.ke ACIDS, BASES A base may be defined as a substance that turn litmus blue. Litmus is a lichen found mainly in West Africa. It changes its colour depending on whether the solution it is in, is basic/alkaline or acidic.It is thus able to identify/show whether An acid is a substance that dissolves in water […] Amino acid - Wikipedia There are three amino acids with side-chains that are cations at neutral pH (though in one, histidine, cationic and neutral forms both exist). They are commonly called basic amino acids, but this term is misleading: histidine can act both as a Brønsted acid and as a Brønsted base at neutral pH, lysine acts as a Brønsted acid, and arginine has a fixed positive charge and does not ionize in ...
EOF
Label each reactant and product in this reaction as a brønsted acid or base h2y
What is the greatest difference between ben jonson's poems 'on my first ... Label each reactant and product in this reaction as a brønsted acid or base. Can someone who is gluten-free drink four lokos? Leave a Comment Cancel reply. Comment. Name Email Website. Save my name, email, and website in this browser for the next time I comment. Search. Search. Label each reactant and product in this reaction as a brønsted acid or ... Bronsted acid: e.g. Tanned base: and . In the given reversible reaction, donates a proton to form and accepts a proton to form . Similarly, on the product side, it can accept a proton to form and it can donate a proton to form. Therefore, e are Bronsted acids and e are Bronsted bases. Step 2 of 3 Acid Base Titration - Amrita Vishwa Vidyapeetham It also indicates that the reverse reaction is negligible and the product C & D are very much more stable than the reactants A & B. Greater the value of K the larger the magnitude of the negative free energy change for the reaction between A & B. ... for instance, when the different oxidation states of the product and reactant produce different ...
Label each reactant and product in this reaction as a brønsted acid or base h2y. Acid Base Titration - Amrita Vishwa Vidyapeetham It also indicates that the reverse reaction is negligible and the product C & D are very much more stable than the reactants A & B. Greater the value of K the larger the magnitude of the negative free energy change for the reaction between A & B. ... for instance, when the different oxidation states of the product and reactant produce different ... Label each reactant and product in this reaction as a brønsted acid or ... Bronsted acid: e.g. Tanned base: and . In the given reversible reaction, donates a proton to form and accepts a proton to form . Similarly, on the product side, it can accept a proton to form and it can donate a proton to form. Therefore, e are Bronsted acids and e are Bronsted bases. Step 2 of 3 What is the greatest difference between ben jonson's poems 'on my first ... Label each reactant and product in this reaction as a brønsted acid or base. Can someone who is gluten-free drink four lokos? Leave a Comment Cancel reply. Comment. Name Email Website. Save my name, email, and website in this browser for the next time I comment. Search. Search.







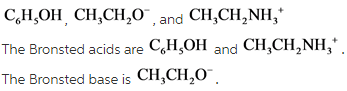











0 Response to "45 label each reactant and product in this reaction as a brønsted acid or base h2y"
Post a Comment